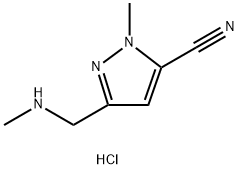
1-methyl-3-((methylamino)methyl)-1H-pyrazole-5-carbonitrilehydrochloride
- русский язык имя
- английское имя1-methyl-3-((methylamino)methyl)-1H-pyrazole-5-carbonitrilehydrochloride
- CAS №1643141-20-1
- CBNumberCB03054477
- ФормулаC7H11ClN4
- мольный вес186.64
- номер MDLMFCD31697852
- файл Mol1643141-20-1.mol
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
1-methyl-3-((methylamino)methyl)-1H-pyrazole-5-carbonitrilehydrochloride химические свойства, назначение, производство
Использование
1-Methyl-3-((methylamino)methyl)-1H-pyrazole-5-carbonitrile hydrochloride can be used as organic synthesis intermediates and pharmaceutical intermediates, mainly used in laboratory research and development processes and chemical production processes.Синтез
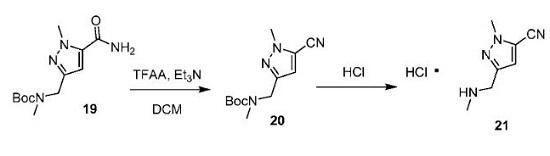 To compound 19 (7.0 kg, 26.1 mol) in DCM (30 L) at O℃ was added triethylamine (5.85
kg, 57.8 mol). The mixture was further cooled to -6°C then trifluoroacetic anhydride (5.85 kg, 27.8
mol) added over 90 minutes [temp kept 0 to 5°C], TLC assay showed the reaction was
incomplete. Additional triethylamine (4.1 kg, 40.5 mol) and trifluoroacetic acid (4.1 kg, 19.5 mol)
10 were added over 2 hours until TLC showed complete reaction. The reaction mixture was
quenched in to water (40 L) [temp to 23°C], The layers were separated and the aqueous re-
extracted with DCM (8 L). The organic layers were sequentially washed with brine (7 L), filtered
through a pad of silica (3 kg) and eluted with DCM (10 L). The filtrate was evaporated and
chromatographed (9 kg silica, eluent 10-30% EtOAc in hexane). Product fractions were
15 evaporated and azeotroped with IPA to afford compound 20 (6.86 kg, ~94 wt%, 25.8 mol, 99%)
as an orange oil. To compound 20 (6.86 kg, ~94 wt%, 25.8 mol) in IPA (35 L) at 17°C was added 37%
hydrochloric acid (6.4 L, 77.4 mol). The mixture was heated to 35°C overnight then concentrated
20 to a moist solid and residual water azeotroped with additional IPA (8 L). The resulting moist solid
was triturated with MTBE (12 L) at 45°C for 30 minutes then cooled to 20°C and filtered, washing
with MTBE (5 L). The solids were dried under vacuum at 45°C to afford compound 21 (4.52 kg,
24.2 mol, 94%) as a white solid. 1H-NMR was consistent with desired product; mp 203-205°C;
HPLC 99.3%.
To compound 19 (7.0 kg, 26.1 mol) in DCM (30 L) at O℃ was added triethylamine (5.85
kg, 57.8 mol). The mixture was further cooled to -6°C then trifluoroacetic anhydride (5.85 kg, 27.8
mol) added over 90 minutes [temp kept 0 to 5°C], TLC assay showed the reaction was
incomplete. Additional triethylamine (4.1 kg, 40.5 mol) and trifluoroacetic acid (4.1 kg, 19.5 mol)
10 were added over 2 hours until TLC showed complete reaction. The reaction mixture was
quenched in to water (40 L) [temp to 23°C], The layers were separated and the aqueous re-
extracted with DCM (8 L). The organic layers were sequentially washed with brine (7 L), filtered
through a pad of silica (3 kg) and eluted with DCM (10 L). The filtrate was evaporated and
chromatographed (9 kg silica, eluent 10-30% EtOAc in hexane). Product fractions were
15 evaporated and azeotroped with IPA to afford compound 20 (6.86 kg, ~94 wt%, 25.8 mol, 99%)
as an orange oil. To compound 20 (6.86 kg, ~94 wt%, 25.8 mol) in IPA (35 L) at 17°C was added 37%
hydrochloric acid (6.4 L, 77.4 mol). The mixture was heated to 35°C overnight then concentrated
20 to a moist solid and residual water azeotroped with additional IPA (8 L). The resulting moist solid
was triturated with MTBE (12 L) at 45°C for 30 minutes then cooled to 20°C and filtered, washing
with MTBE (5 L). The solids were dried under vacuum at 45°C to afford compound 21 (4.52 kg,
24.2 mol, 94%) as a white solid. 1H-NMR was consistent with desired product; mp 203-205°C;
HPLC 99.3%.
1-methyl-3-((methylamino)methyl)-1H-pyrazole-5-carbonitrilehydrochloride поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | |
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| +8615866703830 | China | 8497 | 58 | |
| +86-512-68780025 +8618962125825 |
China | 136 | 58 | |
| +862156762820 +86-13564624040 |
China | 7707 | 58 | |
| +8615255079626 | China | 23541 | 58 | |
| +86-27-50766799 +8618062016861 |
China | 19987 | 58 | |
| +86-0551-65418684 +8618949823763 |
China | 25356 | 58 | |
| +86-19189228086 +8618858568638 |
China | 886 | 58 | |
| +undefined18958062155 | China | 5899 | 58 |