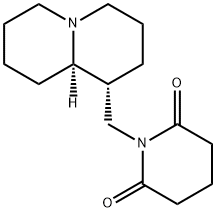
1-[[(1R,8aR)-2,3,4,5,6,7,8,8a-octahydro-1H-quinolizin-1-yl]methyl]pipe ridine-2,6-dione
- русский язык имя
- английское имя1-[[(1R,8aR)-2,3,4,5,6,7,8,8a-octahydro-1H-quinolizin-1-yl]methyl]pipe ridine-2,6-dione
- CAS №18688-40-9
- CBNumberCB01255290
- ФормулаC15H24N2O2
- мольный вес264.36
- номер MDLMFCD28340157
- файл Mol18688-40-9.mol
химическое свойство
| Температура кипения | 418.1±18.0 °C(Predicted) |
| плотность | 1.15±0.1 g/cm3(Predicted) |
| пка | 10.15±0.40(Predicted) |
1-[[(1R,8aR)-2,3,4,5,6,7,8,8a-octahydro-1H-quinolizin-1-yl]methyl]pipe ridine-2,6-dione химические свойства, назначение, производство
Описание
The leaves of Lamprolobium fruticosum yields this alkaloid as a colourless oil, [α]20Do + 29° (EtOH) and furnishing a crystalline picrate, m.p. 153-4°C. Acid hydrolysis of the alkaloid yields glutaric acid and (+ )-l-aminoethylquinolizidine. The stereochemistry of the latter has been established by demonstrating the identity of the N-acetyl derivative, m.p. l44-5°C; [α]20Do + 46° (EtOH) with that prepared from epilupinine. The structure has been confirmed from the infrared, NMR and mass spectra. The total synthesis of the optically inactive alkaloid and its epimer has been carried out.использованная литература
Hart, Johns, Lamberton., Chem. Commun., 302 (1968)Hart, Johns, Lamberton., Austral. J. Chem., 21, 1619 (1968)
Synthesis:
Jeffcoat., Diss. Abstr. Int., 30B, 4973 (1970)
Yamada, Hatano, Matsui., Agr. Bioi. Chem., 34, 1536 (1970)