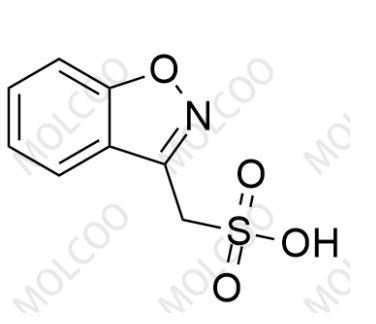
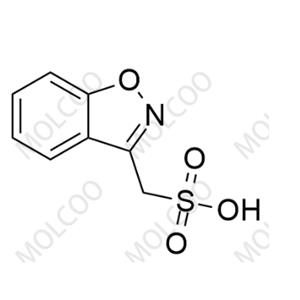
Advantages: As a reference standard for Zonisamide USP Related Compound A, it has a clear chemical structure, confirmed by multiple analytical methods (such as NMR, MS), with a purity of not less than 99.0%. It exhibits good chemical stability and can remain stable for a long time under light-protected and dry storage conditions, with high batch-to-batch quality consistency. It can serve as a reliable reference substance to meet the strict requirements of impurity detection in the quality control of zonisamide bulk drugs and formulations, ensuring accurate, reliable, and reproducible detection results.
Quality Testing: Used to establish and optimize the detection methods for Related Compound A in zonisamide, such as high-performance liquid chromatography (HPLC) and liquid chromatography - mass spectrometry (LC - MS). By accurately determining the content of this impurity, the quality of drugs can be evaluated to meet the standards of pharmacopoeias such as USP.
Process Optimization: During the production of zonisamide, monitor the content of Related Compound A in real time. Analyze its generation under different reaction conditions, optimize the synthesis process, reduce impurity production, and improve the purity and quality of bulk drugs.
Stability Studies: In drug stability tests (accelerated tests, long-term tests), track the changes in the content of Related Compound A, analyze its impact on drug stability, and provide data support for determining the shelf life and storage conditions of drugs.
Regulatory Compliance: Help pharmaceutical companies meet the requirements of domestic and international drug regulatory agencies (such as FDA, EMA, NMPA) for drug impurity limits. Provide accurate impurity detection data during the drug registration and application process to ensure that products meet regulatory standards.
Detection Technology: Currently, the HPLC - UV method is mainly used to detect Zonisamide Related Compound A. By optimizing conditions such as the type of chromatographic column, the composition of the mobile phase, and the detection wavelength, effective separation and accurate quantification of impurities can be achieved, with a detection limit of up to 0.05%. The application of LC - MS technology has further improved the sensitivity and accuracy of detection, enabling more precise identification of impurity structures.
Formation Mechanism: Research shows that Related Compound A may originate from side reactions of the sulfonation process during the synthesis of zonisamide, or from reactions involving impurities in the raw materials. Through in-depth analysis of the synthesis route, the key factors affecting its generation, such as reaction temperature and raw material ratio, have been clarified, providing a theoretical basis for process improvement.
Safety Evaluation: The toxicological research on Related Compound A is gradually being carried out. Preliminary experimental data show that this impurity may affect cell activity at a certain concentration. Therefore, strict limits have been set for it in drug quality standards. In the future, further in-depth research on its toxicity mechanism and safety threshold is needed to develop more scientific and reasonable impurity control strategies.





 China
China