Product Code: T053022
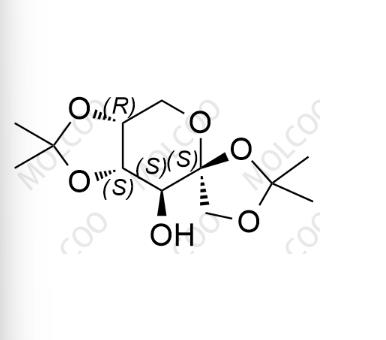
English Name: Topiramate Impurity 22
English Alias: (3aR,4'S,7S,7aS)-2,2,2',2'-tetramethyltetrahydrospiro[[1,3]dioxolo[4,5-c]pyran-6,4'-[1,3]dioxolan]-7-ol
CAS No.: 25018-67-1
Molecular Formula: C₁₂H₂₀O₆
Molecular Weight: 260.28
As an impurity reference standard for Topiramate, it features a clear structure and high purity, which is essential for pharmaceutical quality control and impurity analysis to ensure drug safety and consistency.
The defined stereoconfiguration (3aR,4'S,7S,7aS) makes it suitable for analytical scenarios requiring precise structural characterization.
Primarily used for impurity research, methodological validation (such as development and validation of HPLC, LC-MS, etc.), and formulation of pharmaceutical quality standards for Topiramate raw materials and preparations.
Serves as a reference standard to evaluate the generation and control of impurities in the production process, assisting in optimizing the synthetic route.
Topiramate is a broad-spectrum antiepileptic drug that exerts its effect by blocking sodium channels and enhancing γ-aminobutyric acid (GABA) activity. In the research, development, and production of drugs, impurity control is a key link to ensure drug quality. As a related impurity of Topiramate, Topiramate Impurity 22 may be generated from raw material residues, reaction by-products, or degradation during synthesis, requiring qualitative and quantitative analysis.
Current research on Topiramate impurities mainly focuses on the separation, identification, and toxicity assessment of impurities. The stereostructure of this impurity is relatively complex, and studying its limit standards and detection methods in drugs is the current focus of quality control. With the development of pharmaceutical analysis technologies, highly sensitive and high-resolution analytical methods (such as ultra-high-performance liquid chromatography-mass spectrometry) have been widely used for the precise detection of such impurities, and related studies help improve the quality standards of Topiramate drugs.






 China
China