Tegoprazan Impurity;844648-19-7

WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Code: T058031
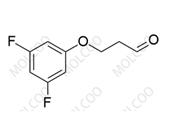
English Name: Tegoprazan Impurity 31
English Alias: 3-(3,5-difluorophenoxy)propanoic acid
CAS No.:844648-19-7
Molecular Formula: C₉H₈F₂O₃
Molecular Weight: 202.15
As an impurity reference standard for Tegoprazan, it features a well-defined structure and high purity, which is essential for the qualitative and quantitative analysis of impurities in drug research, development, and quality control, ensuring the safety and consistency of pharmaceutical products.
Primarily used for impurity detection and quality standard research in the development and production of Tegoprazan-related drugs. By monitoring the content of this impurity, it helps evaluate the stability of the synthesis process and the degradation during storage, providing key references for pharmaceutical quality control.
Tegoprazan is a novel proton pump inhibitor used in the treatment of gastrointestinal diseases. In drug research, development, and manufacturing, impurity control is a critical aspect of ensuring drug quality. Tegoprazan Impurity 31, as a specific impurity of Tegoprazan, requires strict analysis and monitoring to ensure that the drug meets relevant regulatory and standard requirements.
Current research on this impurity mainly focuses on the field of pharmaceutical analysis, including the development of efficient detection methods (such as HPLC, LC-MS, etc.) and the study of its formation mechanisms during drug synthesis and storage. With the increasing requirements for drug impurity control, toxicological studies and the formulation of limit standards for this impurity are also being gradually deepened to ensure the safety of clinical medication.
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com






 China
China