Phosphonylcholine Impurity17364-16-8
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
Product Information
Product Number: P096005
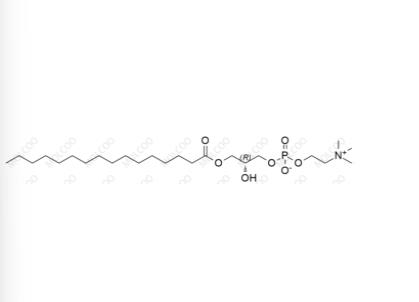
English Name: Phosphonylcholine Impurity 5
English Alias: (R)-2-hydroxy-3-(palmitoyloxy)propyl (2-(trimethylammonio)ethyl) phosphate
CAS Number: 17364-16-8
Molecular Formula: C24H50NO7P
Molecular Weight: 495.63
Advantages: As a reference standard for Phosphonylcholine Impurity 5, it has a clear chemical structure, high purity, and good stability. Its precise quality control ensures that it can serve as a reliable reference in impurity detection and analysis, effectively guaranteeing the accuracy and repeatability of detection results, and meeting the strict requirements of scientific research, pharmaceutical and other fields for impurity research and drug quality control.
Applications: It is mainly used in the quality research and control of phosphonylcholine and its related drugs and materials. In drug research and development, it is used to establish and validate impurity detection methods, such as high-performance liquid chromatography (HPLC) and liquid chromatography - mass spectrometry (LC - MS), to determine the content of this impurity in drugs. During the production process, it is used to monitor product quality to ensure that phosphonylcholine products meet quality standards. It can also be used to study the impact of this impurity on the performance, stability and safety of phosphonylcholine-related products, providing a basis for optimizing the production process.
Background Description: Phosphonylcholine is widely used in the fields of drugs and biomaterials, and the quality of its products directly affects the effectiveness and safety of related applications. In the production and research and development process of phosphonylcholine, the presence of impurities may interfere with product performance and even pose potential risks. As one of the impurities, in-depth research and strict control of Phosphonylcholine Impurity 5 help to improve the quality of phosphonylcholine products and ensure the reliability of related applications.
Research Status: Currently, the research on Phosphonylcholine Impurity 5 focuses on optimizing impurity detection techniques to improve detection sensitivity and accuracy, so as to achieve precise determination of trace impurities. At the same time, the generation mechanism of this impurity in the production process is being explored, and the process is being improved to reduce its generation. In addition, the research on the impact of this impurity on the performance and safety of phosphonylcholine products is also gradually underway, in order to more comprehensively evaluate its role in product quality and provide a more scientific basis for quality control.
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!






 China
China


