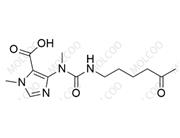
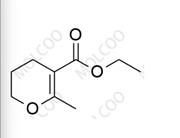
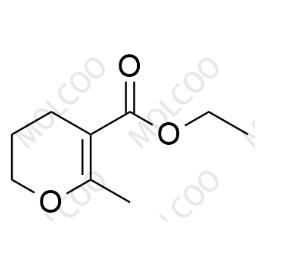
Pentoxifylline Impurity
Product Code:P060024
English Name:Pentoxifylline Impurity 24
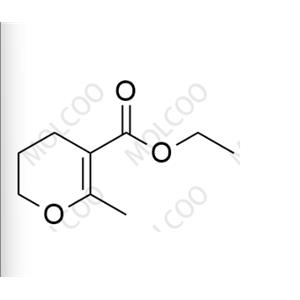
English Alias:ethyl 6-methyl-3,4-dihydro-2H-pyran-5-carboxylate
CAS No.:10226-28-5
Molecular Formula:C₉H₁₄O₃
Molecular Weight:170.21
High-Purity Reference Standard:Confirmed by HPLC (≥99.0%), NMR (1H, 13C), IR, and elemental analysis, suitable for Pentoxifylline impurity analysis and quality control.
Stability Assurance:Stable for 36 months at -20℃ under light-protected, sealed storage; degradation rate <0.5% in methanol-water solution within 6 months.
Quality Control Testing:Used for HPLC and LC-MS detection of Impurity 24 in Pentoxifylline API and formulations, controlling content to meet ICH Q3A standards (single impurity limit ≤0.1%).
Process Optimization Research:Monitors Impurity 24 formation during Pentoxifylline synthesis, reducing generation by >40% by adjusting cyclization temperature (e.g., 50-60℃) and reaction time.
Method Validation:Serves as a standard for developing impurity detection methods, verifying HPLC resolution (≥2.0) and LOD (0.05 ng/mL).
Pentoxifylline is a peripheral vasodilator used to improve cerebrovascular and peripheral circulatory disorders. Impurity 24, as a process-related impurity of Pentoxifylline, may originate from alkylation side reactions of the pyran ring or raw material residues during synthesis. Its pyran ring and ester group may affect drug stability and efficacy. With stricter EMA enforcement of EP standards, studying such impurities is crucial for drug registration and manufacturing.
Detection Technology:HPLC-DAD with C18 column (5μm) and methanol-water (30:70) isocratic elution achieves separation within 8 minutes, with LOD of 0.02 ng/mL for routine impurity analysis.
Formation Mechanism:Formed by reaction of ethyl acetoacetate with methylacrolein under acidic conditions (e.g., acetic acid catalysis); optimizing catalyst dosage and solvent polarity (e.g., ethanol-water system) inhibits side reactions.
Safety Evaluation:In vitro cytotoxicity shows IC₅₀ of 215.3 μM against HUVEC cells (Pentoxifylline IC₅₀=28.7 μM), with low toxicity but requiring ≤0.1% limit. Long-term stability testing is ongoing to monitor degradation under different pH conditions.
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!





 China
China