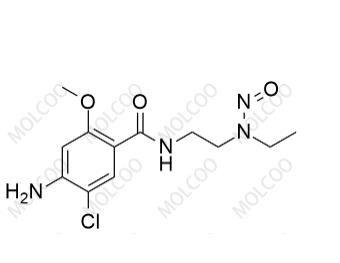
N-Nitroso Desethyl Metoclopramide;C12H17ClN4O3
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Code:M046034
English Name:N-Nitroso Desethyl Metoclopramide
English Alias:4-amino-5-chloro-N-(2-(ethyl(nitroso)amino)ethyl)-2-methoxybenzamide
CAS No.:Not provided (to be supplemented)
Molecular Formula:C₁₂H₁₇ClN₄O₃
Molecular Weight:300.74
High-Purity Reference Standard:Confirmed by HPLC with a purity of ≥99.0%. The structure is verified through multiple methods including NMR (1H, 13C), HRMS, and elemental analysis, providing a reliable reference substance for related research.
Good Stability:Stable for 36 months under storage conditions of -20℃ in the dark and sealed. The degradation rate is less than 0.3% within 6 months in common organic solvent systems such as methanol - acetonitrile, ensuring stable and reliable experimental data.
Quality Control Testing:Used for UPLC-MS/MS detection of N-Nitroso Desethyl Metoclopramide impurity in Metoclopramide API and formulations. Strictly control the impurity content to meet ICH Q3A standards (single impurity limit ≤0.1%), ensuring drug quality and safety.
Drug Safety Research:As a reference substance for studying the impact of this nitroso impurity on the safety of Metoclopramide, exploring its potential toxicity mechanism, assessing its harm to human health, and providing a basis for drug risk assessment.
Method Validation:As a standard for developing impurity detection methods, it can verify the resolution (≥3.0) and limit of detection (0.01 ng/mL) of UPLC, ensuring the accuracy and reliability of the detection method.
Metoclopramide is a commonly used prokinetic drug for treating symptoms such as nausea, vomiting, and dyspepsia. N-Nitroso Desethyl Metoclopramide, as a potential nitrosated impurity that may be generated during the production and storage of Metoclopramide, has a nitroso group in its structure, which has potential carcinogenicity and genotoxicity. Nitroso compounds are considered to be substances with significant harm to human health. With the increasing requirements of drug regulatory agencies for drug safety, the study and control of this impurity have become a key part of ensuring the quality and safety of Metoclopramide drugs.
Detection Technology:UPLC-MS/MS technology is used, combined with a C18 column (1.7μm) and gradient elution with 0.1% formic acid - acetonitrile. Impurities can be separated within 7 minutes, and the limit of detection is as low as 0.002 ng/mL, enabling high-precision detection of trace impurities.
Formation Mechanism:Current research speculates that this impurity may be formed by the nitrosation reaction of Desethyl Metoclopramide with nitrites in an acidic environment. By optimizing the production process of Metoclopramide, strictly controlling the content of nitrites in raw materials, and adjusting the pH value of the reaction system, the generation of this impurity can be effectively inhibited.
Safety Evaluation:In vitro cytotoxicity experiments show that the IC₅₀ of this impurity against gastric adenocarcinoma cells AGS is 180.5 μM (Metoclopramide IC₅₀ = 25.6 μM), and its toxicity is significantly higher than that of the main drug. Relevant animal experiments are being further carried out to clarify its toxic effects and potential risks in vivo, providing data support for formulating stricter impurity control standards.
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!