Product Number: N031282
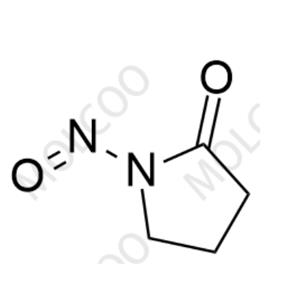
English Name: N-Nitroso-2-Pyrrolidinone
English Alias: 1-nitrosopyrrolidin-2-one
CAS Number: 54634-49-0
Molecular Formula: C₄H₆N₂O₂
Molecular Weight: 114.10
Product Advantages:
High purity and structural confirmation:HPLC purity ≥99.0%, with structure verified by 1H NMR, 13C NMR, and HRMS, meeting strict requirements of FDA and WHO for nitrosamine impurity reference standards.
Controllable stability:Stable for 24 months when stored at -20°C in the dark, with a degradation rate <1% after 7 days at room temperature in solution (e.g., acetonitrile-water system), suitable for long-term quality monitoring and stability studies.
Specific identification:As a typical N-nitroso compound, it accurately tracks risks of nitrosation side reactions during pharmaceutical production, helping companies identify process hazards.
Applications:
Nitrosamine impurity detection in pharmaceuticals:Used for LC-MS/MS detection of nitrosamine impurities in drugs like sartans and ranitidine, controlling content according to ICH M7(R1) standards to ensure compliance with genotoxic impurity (GTIs) regulations.
Process risk assessment:In API synthesis and pharmaceutical packaging migration studies, monitoring impurity content optimizes nitrosation conditions or screens low-risk excipients to reduce nitrosamine formation.
Analytical method development:Serves as a standard reference for establishing specific detection methods, such as ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS), achieving a detection limit of 0.1 ng/mL for trace analysis.
Toxicological research support:Provides samples for evaluating the potential carcinogenicity of nitrosamines, facilitating in vitro Ames tests and in vivo animal toxicity studies to support pharmaceutical safety assessments.
Background Description:
N-Nitroso-2-Pyrrolidinone belongs to N-nitroso compounds (NOCs), a class of known potential carcinogens. In medications like sartan antihypertensives and ranitidine, such impurities may be introduced due to raw material residues, process defects, or packaging material migration. Since 2018, global regulatory agencies (e.g., FDA, EMA) have issued guidelines mandating strict control of nitrosamine impurities in drugs, driving research on detection and control technologies.
Research Status:
Advances in detection technology:UPLC-MS/MS with multiple reaction monitoring (MRM) is the mainstream method, enabling ultra-trace detection of N-Nitroso-2-Pyrrolidinone with a limit of quantitation (LOQ) as low as 0.01 ng/mL. GC-MS/MS methods, after derivatization, also enhance detection sensitivity significantly.
Formation mechanism research:This impurity mainly results from reactions between secondary amines (e.g., pyrrolidinone) and nitrosating agents (e.g., sodium nitrite). Acidic conditions (pH<4), high temperatures (>60℃), or metal ion catalysis accelerate its formation. Optimizing reaction conditions, using chelating agents, or selecting non-nitrosating process routes can reduce impurity generation.
Safety evaluation:Animal studies show that long-term exposure to N-Nitroso-2-Pyrrolidinone can induce liver and lung tumors, and it is classified as a Group 2A possible carcinogen by the IARC. Current regulatory requirements limit its content in drugs to no more than the acceptable intake (AI) of 1.5 μg per day.





 China
China