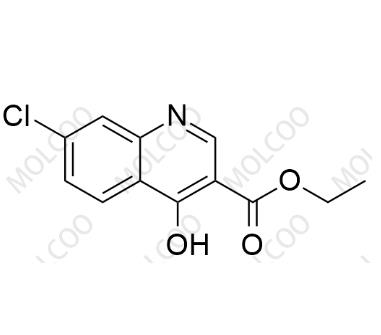
Product Number: L021080
English Name: Lenvatinib Impurity 80
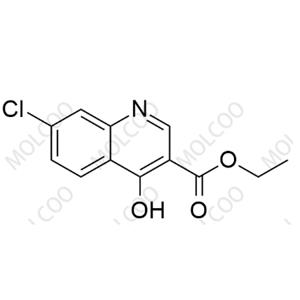
English Alias: ethyl 7-chloro-4-hydroxyquinoline-3-carboxylate
CAS Number: 16600-22-9
Molecular Formula: C₁₂H₁₀ClNO₃
Molecular Weight: 251.67
As an impurity of lenvatinib, Lenvatinib Impurity 80 has a well-defined chemical structure and stable properties, and can be used as a standard reference for drug research, development, and quality control. Its presence helps to analyze the possible side reaction mechanisms during lenvatinib synthesis. Through precise impurity reference analysis, it can optimize the production process, improve product quality, and ensure that drug safety and effectiveness meet high standards.
Drug Development: In the research and development of lenvatinib and its formulations, it is used as an impurity reference standard for identification and quantitative analysis, helping to determine the impurity profile of drugs and evaluate the purity of active pharmaceutical ingredients and formulations;
Quality Control: As a standard substance, it is used to verify the accuracy and sensitivity of detection methods such as HPLC and LC-MS, ensuring that impurity content meets pharmacopoeia and regulatory requirements during production;
Stability Studies: Research on the stability of this impurity under different conditions provides data support for determining the storage conditions and shelf life of lenvatinib.
Lenvatinib is a multi-target tyrosine kinase inhibitor widely used in the treatment of various cancers such as liver cancer and thyroid cancer. During drug synthesis and production, various impurities are inevitably generated due to factors such as reaction conditions and raw material purity. As one of these impurities, the content and properties of Lenvatinib Impurity 80 directly affect the quality of lenvatinib drugs. With the increasing requirements for drug quality and safety in the pharmaceutical industry, the research and control of this impurity have become an important part of the lenvatinib quality control system.
Currently, research on Lenvatinib Impurity 80 mainly focuses on the development and optimization of impurity detection methods, improvement of synthesis processes, and toxicological evaluations. Researchers develop more sensitive and accurate detection technologies, such as ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS), to achieve trace detection of this impurity. At the same time, they deeply study its synthesis mechanism and optimize the production process of lenvatinib to reduce impurity generation. In addition, in vitro cell experiments and animal models are used to evaluate the potential toxicity of this impurity, providing a scientific basis for formulating reasonable impurity limit standards





 China
China