Product Number: F036014
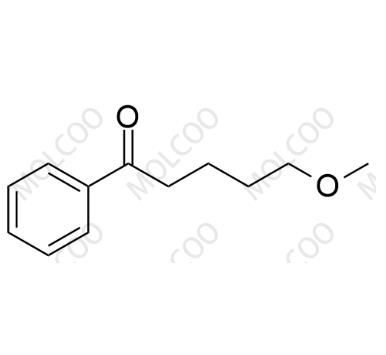
English Name: Fluvoxamine Impurity 14
English Alias: 5-methoxy-1-phenylpentan-1-one
CAS Number: 34008-70-3
Molecular Formula: C₁₂H₁₆O₂
Molecular Weight: 192.25
As an impurity reference standard for fluvoxamine, this compound has the following advantages:
Well-defined structure and high stability, which can be used to analyze the by-product formation mechanism of etherification and oxidation reactions in fluvoxamine synthesis, optimizing processes to control ketone impurity generation;
As a reference standard containing methoxy and benzene ring, it provides a standard substance for detecting aromatic ketone impurities in drugs, improving the quantitative accuracy of methods such as HPLC;
Helps study the impact of methoxy substituents on drug stability and toxicological properties to provide a scientific basis for impurity control strategies.
Drug Development: Used as an impurity reference standard to identify and quantify Impurity 14 in fluvoxamine preparations, evaluating the purity of APIs and formulations;
Quality Control: Acting as a standard substance to validate the sensitivity of detection methods (e.g., HPLC or LC-MS), ensuring the impurity content meets pharmacopoeia requirements during production;
Stability Studies: Investigating the formation rate of this impurity under light and high-temperature conditions to evaluate its impact on fluvoxamine formulation stability.
Fluvoxamine is a selective serotonin reuptake inhibitor (SSRI) used for treating depression and obsessive-compulsive disorder. If the methoxylation reaction is incomplete or intermediate products are oxidized during its synthesis, ketone impurities such as 5-methoxy-1-phenylpentan-1-one are easily generated. Since ketone compounds may affect the safety and effectiveness of drugs, research on this impurity is an important part of fluvoxamine quality control.
Current research focuses on:
Detection Method Optimization: Establishing highly sensitive detection methods for this impurity using HPLC-UV or LC-MS combined techniques to achieve trace analysis;
Synthesis Process Improvement: Reducing by-product generation by optimizing the catalyst and reaction temperature of methoxylation reactions;
Toxicological Evaluation: Studying the potential toxicity of this ketone structure through in vitro cell experiments to provide data support for establishing safe limits;
Stability Studies: Investigating the degradation behavior of this impurity under different storage conditions to evaluate its impact on fluvoxamine formulation stability.





 China
China