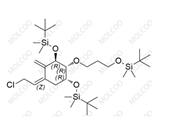
Product Number: E034003
English Name: Eldecalcitol Impurity 3
English Alias: (((1R,2R,3R,Z)-2-(3-((tert-butyldimethylsilyl)oxy)propoxy)-5-((E)-2-((1R,3aS,7aR)-7a-methyl-1-((R)-6-methyl-6-((trimethylsilyl)oxy)heptan-2-yl)hexahydro-1H-inden-4(2H)-ylidene)ethylidene)-4-methylenecyclohexane-1,3-diyl)bis(oxy))bis(tert-butyldimethylsilane)
CAS Number: 342645-84-5
Molecular Formula: C₅₁H₁₀₀O₅Si₄
Molecular Weight: 905.68
As an impurity of Eldecalcitol, this compound has the following advantages:
Well-defined and distinct structure: Contains steroidal skeleton, multiple silyl protecting groups (TBS, TMS), methylene, and double bonds, differing from eldecalcitol by retained silyl ethers (unremoved hydroxyl protection). Strong hydrophobicity from polysilyl groups enables clear differentiation via GC or normal-phase HPLC, serving as a specific impurity marker;
High stability and traceability: Silyl ethers shield hydroxyls for stability under non-acidic conditions. As an intermediate from incomplete desilylation in eldecalcitol synthesis, it directly reflects deprotection efficiency, improving process tracing accuracy;
High detection sensitivity: UV absorption (250-270nm) from conjugated steroidal double bonds, combined with silicon-specific mass responses (e.g., Si-CH₃ fragments), enables trace analysis (ppm level) via GC-MS or LC-MS, compatible with silyl-protected vitamin D derivative systems.
Pharmaceutical quality control: Used as a reference standard to quantify Eldecalcitol Impurity 3 in API production, ensuring residual silylated intermediates meet quality standards post-desilylation;
Synthesis optimization: Optimizing desilylation conditions (fluoride reagent dosage, temperature) by monitoring impurity levels to enhance hydroxyl deprotection efficiency;
Intermediate purity assessment: Evaluating purity of key silylated intermediates in eldecalcitol synthesis to support specificity of downstream reactions.
Eldecalcitol, an active vitamin D₃ analog for osteoporosis, requires silyl protection/deprotection of hydroxyl groups in synthesis. Incomplete removal of silyl ethers (e.g., TBS) may generate polysilylated derivatives like Eldecalcitol Impurity 3. Its presence risks affecting water solubility and bioactivity, making control critical for eldecalcitol quality assurance.
Current research focuses on:
Analytical method validation: Developing GC-MS assays with silicon isotope monitoring for separation/quantification of impurity and eldecalcitol, achieving 0.5 ppm detection limits;
Desilylation kinetics: Studying impurity formation under varying fluoride concentrations to clarify silyl ether cleavage mechanisms;
Scale-up process control: Implementing impurity monitoring in kg-scale production to optimize desilylation parameters for consistent quality;
Structural characterization: Using NMR and X-ray diffraction to confirm stereochemistry, supporting structural differentiation from eldecalcitol.





 China
China