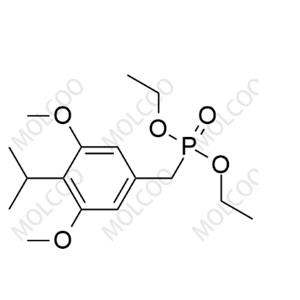
Benvitimod Impurity 11;443982-76-1

WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Number: B051011
English Name: Benvitimod Impurity 11
English Alias: diethyl 4-isopropyl-3,5-dimethoxybenzylphosphonate
CAS Number: 443982-76-1
Molecular Formula: C16H27O5P
Molecular Weight: 330.36
Product Advantages: Benvitimod Impurity 11 has high purity and good chemical stability. With a clear structure and uniform properties, it remains stable under different experimental conditions and can be used as a reliable reference substance for Benvitimod impurity analysis. Its precise characteristics can ensure the accuracy and repeatability of detection results, providing a solid basis for pharmaceutical quality research and quality control.
Application Fields: It is mainly applied in the quality control and research and development process of Benvitimod-related drugs. As an impurity reference standard, it is used to establish and validate the detection methods of Benvitimod impurities, ensuring the sensitivity and specificity of detection methods. During the drug production process, it is used to monitor the content of this impurity, assist in optimizing the production process, and prevent excessive impurities from affecting drug quality. In the study of drug stability, it analyzes its changes during storage, providing important references for determining the shelf life and storage conditions of drugs.
Background Description: Benvitimod is a drug for the treatment of skin diseases such as psoriasis. In its research, development, production and quality control, impurity research is of great significance. The presence of impurities may affect the safety, effectiveness and stability of drugs. As a related impurity of Benvitimod, in-depth research on Benvitimod Impurity 11 helps to comprehensively evaluate the quality of Benvitimod drugs and ensure the safety and effectiveness of clinical medication.
Research Status: Currently, research on Benvitimod Impurity 11 continues to advance. In terms of detection technology, advanced methods such as Ultra-Performance Liquid Chromatography-Mass Spectrometry (UPLC-MS) and high-resolution mass spectrometry are constantly being explored to achieve accurate detection of trace impurities. In the study of impurity generation mechanisms, the formation causes and influencing factors are deeply analyzed by simulating drug synthesis reactions and storage environments, providing theoretical support for controlling impurities from the source. At the same time, research on the interaction between this impurity and Benvitimod and its impact on drug efficacy and safety is also gradually carried out, aiming to improve the comprehensive understanding of the quality of Benvitimod drugs.
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!





 China
China