Product Number: A076007
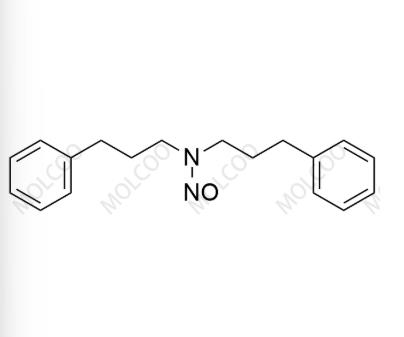
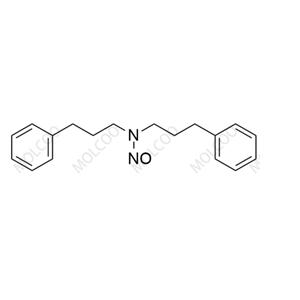
English Name: Alverine Nitroso Impurity 7
English Alias: N,N-bis(3-phenylpropyl)nitrous amide
CAS Number: 871884-77-4
Molecular Formula: C₁₈H₂₂N₂O
Molecular Weight: 282.38
As a nitroso impurity reference standard for alverine, this compound has the following advantages:
Well-defined structure and high stability, which can be used to analyze the by-product formation mechanism of nitrosation reactions during alverine synthesis or storage, optimizing processes to control nitroso impurity generation;
Serving as a reference standard containing nitroso and bis(phenylpropyl) structures, providing a standard substance for detecting nitroso impurities in drugs, and helping to evaluate drug safety (nitroso compounds may have potential carcinogenicity);
Helping study the impact of nitroso structures on drug stability and toxicological properties to provide a scientific basis for formulating impurity control strategies.
Drug Development: Used as an impurity reference standard to identify and quantify N-nitroso impurities in alverine preparations, evaluating the purity of APIs and formulations;
Quality Control: Acting as a standard substance to validate the sensitivity of detection methods (e.g., HPLC or LC-MS), ensuring nitroso impurity content meets ICH guideline requirements during production;
Toxicological Research: Assisting in evaluating the potential genotoxicity of nitroso impurities to provide data support for drug safety evaluation.
Alverine is a smooth muscle relaxant used for relieving gastrointestinal spasms. Due to the potential carcinogenic risk of nitroso compounds (such as N-nitrosamines), drug regulatory authorities worldwide have put forward strict control requirements for nitroso impurities in drugs. Alverine Nitroso Impurity 7, as an impurity with a nitroso structure in alverine, may be generated during synthesis, storage, or metabolism. Therefore, research on it is a key link in drug quality control and safety assessment.
Current research focuses on:
Synthesis Methods: Developing high-purity synthesis processes for Alverine Nitroso Impurity 7, solving the challenge of poor stability of nitroso compounds to meet the needs of toxicological research and quality control;
Detection Technologies: Establishing trace detection methods for nitroso impurities (detection limits reach ppb level) using ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) and other technologies;
Toxicological Evaluation: Studying the potential mutagenicity and carcinogenicity of this impurity through in vitro Ames tests and animal models;
Process Control: Analyzing the inducements (such as raw material residues, reaction conditions) of nitrosation reactions to optimize the synthesis route or storage conditions to reduce the generation of nitroso impurities.





 China
China