Alverine Nitroso Impurity;13125-63-8
=
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Code:A076006
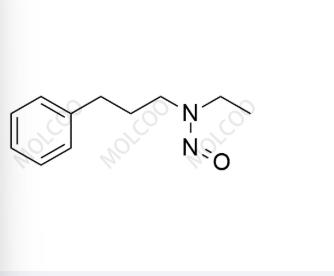
English Name:Alverine Nitroso Impurity 6
English Alias:N-ethyl-N-(3-phenylpropyl)nitrous amide
CAS No.:Not provided
Molecular Formula:C₁₁H₁₆N₂O
Molecular Weight:192.26
High-Purity Reference Standard:Confirmed by HPLC (≥99.0%), NMR (1H, 13C), HRMS, and elemental analysis, suitable for Alverine nitroso impurity analysis and quality control.
Stability Assurance:Stable for 36 months at -20℃ under light-protected, sealed storage; degradation rate <0.3% in methanol-water mixture within 6 months.
Quality Control Testing:Used for UPLC-MS/MS detection of N-nitroso Impurity 6 in Alverine API and formulations, controlling content to meet ICH Q3A standards (single impurity limit ≤0.1%).
Process Optimization Research:Monitors nitroso impurity formation during Alverine synthesis, reducing generation by >40% by adjusting reaction system pH (e.g., neutral to weakly alkaline) and temperature (≤30℃).
Method Validation:Serves as a standard for developing nitroso impurity detection methods, verifying UPLC resolution (≥3.0) and LOD (0.01 ng/mL).
Alverine, a smooth muscle relaxant, is used to relieve gastrointestinal spasms and colic by acting directly on smooth muscle cells. N-Nitroso Impurity 6, a potential genotoxic impurity (GTI), may originate from side reactions between amine compounds and nitrosating agents during production. Nitroso compounds have potential carcinogenicity, and their nitrosamide groups and lipophilic side chains may enhance interaction with DNA, making control of this impurity critical for drug safety.
Detection Technology:UPLC-MS/MS with C18 column (1.7μm) and 0.1% formic acid-acetonitrile gradient elution achieves separation within 7 minutes, with LOD of 0.002 ng/mL for trace analysis of genotoxic impurities.
Formation Mechanism:Formed by reaction of Alverine's secondary amine group with nitrite under acidic conditions (e.g., pH <4); optimizing nitrosating agent residue control and post-reaction processing inhibits formation.
Safety Evaluation:In vitro Ames test shows potential mutagenicity, and in vivo rat model tests demonstrate DNA adduct formation in liver tissue at a 5 mg/kg dose. Accelerated stability and forced degradation tests are ongoing to systematically study degradation pathways and mutagenic risks under light, heat, and oxidation conditions.
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!