Cetylpyridinium Chloride Impurity;4860-03-1
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Code: C141002
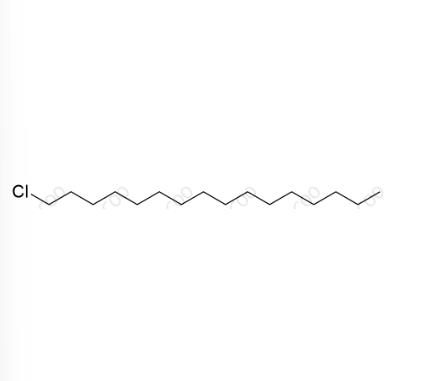
English Name: Cetylpyridinium Chloride Impurity 2
English Alias: 1-chlorohexadecane
CAS No.:4860-03-1
Molecular Formula: C₁₆H₃₃Cl
Molecular Weight: 260.89
As an impurity standard of cetylpyridinium chloride, it has a clear structure and high purity, which is suitable for qualitative and quantitative analysis of drug impurities to ensure the accuracy of pharmaceutical quality control.
It has stable chemical properties, is easy to store and use, and is applicable to quality detection in laboratory research and pharmaceutical industry.
Mainly used for impurity research, analytical method validation, and quality control of cetylpyridinium chloride-related drugs, such as detecting impurity content in drug research and development and production processes to ensure that drugs meet pharmacopoeia and regulatory requirements.
Can be used as a reference standard for chromatographic analysis (such as HPLC, GC), spectroscopic analysis, etc., to assist in determining the type and concentration of impurities in drugs.
Cetylpyridinium chloride is a cationic surfactant commonly used in oral care products (such as mouthwashes), topical bactericides, and pharmaceutical preparations, with antibacterial and bacteriostatic effects. Impurities such as 1-chlorohexadecane may be generated during its synthesis or storage. The presence of impurities may affect the safety and effectiveness of drugs, so the control of impurities is an important part of drug quality research. As one of its impurities, 1-chlorohexadecane needs to be systematically studied through standard substances to establish a complete quality control system.
At present, the research on impurities of cetylpyridinium chloride mainly focuses on the analysis of impurity sources, the development of detection methods, and the setting of limits. For 1-chlorohexadecane, quantitative analysis by gas chromatography (GC) or liquid chromatography and other methods have been reported in the literature to study its formation mechanism and removal process in drugs. With the improvement of drug regulatory standards, the research on this impurity is developing towards more sensitive and precise detection technologies to meet the requirements of international pharmacopoeias (such as USP, EP, CP) for impurity control.
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!





 China
China