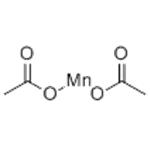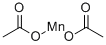
Manganese(II) acetate
- Product Name Manganese(II) acetate
- CAS638-38-0
- MFC4H6MnO4
- MW173.03
- EINECS211-334-3
- MOL File638-38-0.mol
Chemical Properties
| Melting point | 180°C |
| Density | 1.589 |
| vapor pressure | 13hPa at 25℃ |
| storage temp. | Store below +30°C. |
| form | Powder |
| color | Grayish-beige to light brown |
| Water Solubility | Soluble in water, methanol and acetic acid. |
| Sensitive | Hygroscopic |
| Merck | 13,5746 |
| Exposure limits | OSHA: Ceiling 5 mg/m3 NIOSH: IDLH 500 mg/m3; TWA 1 mg/m3; STEL 3 mg/m3 |
| InChI | InChI=1S/2C2H4O2.Mn/c2*1-2(3)4;/h2*1H3,(H,3,4);/q;;+2/p-2 |
| InChIKey | NKKRYPIWDQMINN-UHFFFAOYSA-M |
| SMILES | C(=O)(C)O[Mn]OC(=O)C |
| CAS DataBase Reference | 638-38-0(CAS DataBase Reference) |
| EPA Substance Registry System | Acetic acid, manganese(2+) salt (638-38-0) |
Safety Information
| Hazard Codes | Xn |
| Risk Statements | 20/21/22-36/37/38-40 |
| Safety Statements | 26-27-36/37/39 |
| WGK Germany | 3 |
| RTECS | AI5770000 |
| TSCA | TSCA listed |
| HS Code | 2915 29 00 |
| Storage Class | 11 - Combustible Solids |
| Hazard Classifications | Aquatic Chronic 3 STOT RE 2 Inhalation |
| Hazardous Substances Data | 638-38-0(Hazardous Substances Data) |