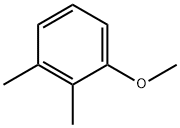
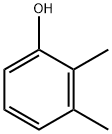
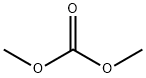
General procedure: 1-methoxy-2,3-dimethylbenzene was synthesized by alkylation of 2,3-dimethylphenol with dimethyl carbonate. The specific operation was as follows: 3 mmol of Mn2(CO)10, W(CO)6 or Co2(CO)8 catalyst, 100 mmol of 2,3-dimethylphenol, and 300 mmol of dimethyl carbonate were sequentially added to a 17 mL stainless steel high-pressure microreactor. After sealing the reactor, the reaction was heated at 180 °C for 1 hour. Upon completion of the reaction, the reactor was cooled to room temperature, opened and the reaction mixture was filtered through an alumina layer. The unreacted dimethyl carbonate was removed by distillation, and the residue was distilled under atmospheric or reduced pressure conditions or recrystallized using ethanol to obtain the target product 1-methoxy-2,3-dimethylbenzene. The yield of the reaction was 99% and the boiling point of the product was 95.8-96 °C (30 mmHg).13C NMR data (δC, ppm): 11.99 (CH3), 20.56 (CH3), 55.83 (OCH3), 110.02 (C6), 122.43 (C4), 124.78 (C2), 128.74 (C5). 137.70 (C3), 157.36 (C1). Elemental analysis results (measured values): C 79.29%, H 8.82%; calculated values for molecular formula C9H12O: C 79.37%, H 8.88%.