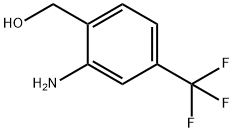
2-Amino-4-(trifluoromethyl)benzenemethanol
- Product Name2-Amino-4-(trifluoromethyl)benzenemethanol
- CAS186602-93-7
- MFC8H8F3NO
- MW191.15
- EINECS
- MOL File186602-93-7.mol
Chemical Properties
| Boiling point | 280.0±40.0 °C(Predicted) |
| Density | 1.374±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| pka | 13.79±0.10(Predicted) |