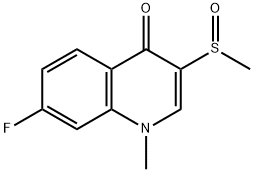
Flosequinan is a new mixed arteriolar and venous vasodilator useful for the treatment
of patients with congestive heart failure with or without ACE inhibitors, but especially
in those patients who are intolerant to ACE inhibitors. It does not cause reflex
stimulation of the renin-angiotensin or sympathetic nervous system. How flosequinan
achieves its direct vasodilating effect is not clear, it is thought to interfere with protein
kinase C and the production of inositol triphosphate. Flosequinan is well absorbed
orally, and with the relatively long half-life of its sulfone metabolite it is suitable for
once a day administration.
7-Fluoro-3-methylsulphinyl-4-quinolone (5.0 g) was dissolved in hot butanone
(250 ml) containing anhydrous potassium carbonate (3.06 g). The resulting
suspension was stirred and treated dropwise with dimethyl sulphate (2.09 ml).
The mixture was stirred and boiled under reflux for 1 hour and filtered while
hot. The filtrate was allowed to cool, giving a crystalline product. The product
was collected and dried to give 7-fluoro-1-methyl-3-methylsulphinyl-4-quinolone, melting point 226-228°C.
The intermediate 7-fluoro-3-methylsulphinyl-4-quinolone, was prepared in the
following way:
A solution of 2-amino-4-fluorobenzoic acid (62 g) in aqueous sodium
carbonate (44 g sodium carbonate in 1.6 liters water) was stirred and treated
dropwise with a solution of phosgene (120 g) in toluene (500 ml) during 1.5
hours. The resulting suspension was stirred at room temperature for 24 hours.
The solid product was collected by filtration, washed with water and dried to
give 7-fluoro-1,2-dihydro-3,1(4H)-benzoxazine-2,4-dione; melting point 217-
219°C.
A mixture of dimethyl sulphoxide (230 ml), toluene (300 ml) and 50% w/w
dispersion of sodium hydride in mineral oil (20.7 g) was heated under
nitrogen at 65-70°C for 1 hour, then cooled to room temperature to form
dimethylsulphoxide anion, sodium salt. The resulting suspension was stirred
under nitrogen and the above benzoxazine-2,4-dione (27.5 g) was added
portionwise. The resulting solution was stirred at room temperature for 15
minutes and then poured into ether (3 liters). The resulting solid was collected
by filtration and dissolved in water (300 ml) and the solution acidified with
glacial acetic acid to a final pH of 6.0. The solution was saturated with solid
potassium carbonate. The resulting precipitate was collected, dried and
recrystallised from ethanol/diethyl ether to give the novel compound 2'-
amino-4'-fluoro-(2-methylsulphinyl)acetophenone, melting point 115-117°C.
This compound (14 g) was dissolved in triethyl orthoformate (160 ml) at
100°C under nitrogen. The resulting solution was treated dripwise with
piperidine (7 ml). The mixture was heated with stirring at 120°C under
nitrogen for 30 min allowing ethanol produced to distil off, then cooled to
room temperature. The solid product was collected, dried and crystalized from
ethanol using charcoal to give the 7-fluoro-3-methylsulphinyl-4-quinolone;
melting point 265°C.