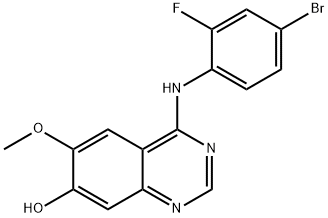
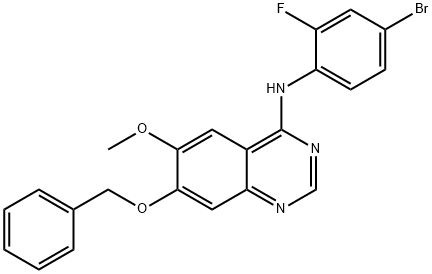
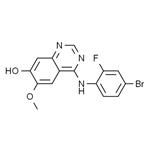
General procedure for the synthesis of 4-(4-bromo-2-fluoroanilino)-7-hydroxy-6-methoxyquinazoline from 7-(benzyloxy)-N-(4-bromo-2-fluorophenyl)-6-methoxyquinazolin-4-amine: the intermediate 7-(benzyloxy)-N-(4-bromo-2-fluorophenyl)-6-methoxyquinazolin-4-amine (1.30 g, 2.86 mmol) was dissolved in trifluoroacetic acid (15 mL) and the solution was heated to reflux for 1.5 hours. Upon completion of the reaction, the solution was cooled to room temperature and concentrated under reduced pressure. Methanol (20 mL) was added to the residual brown solid and the pH was adjusted with concentrated ammonium hydroxide solution to 11. The mixture was concentrated under reduced pressure and dried under high vacuum, and then purified by fast column chromatography on a silica gel column (20 g) with the eluent being a dichloromethane solution of 5-20% methanol. This step afforded the target product 4-(4-bromo-2-fluoroanilino)-7-hydroxy-6-methoxyquinazoline (1.03 g, 99% yield) as a light yellow solid.1H NMR (300 MHz, DMSO-d6): δ 10.5 (br s, 1H), 9.53 (br s, 1H), 8.34 (s, 1H), 7.81 (s, 1H). 7.63 (d, 1H, J = 9.6 Hz), 7.54 (dd, 1H, J = 8.4, 7.8 Hz), 7.44 (d, 1H, J = 8.4 Hz), 7.12 (s, 1H), 3.95 (s, 3H).13C NMR (75 MHz, DMSO-d6): δ 156.77, 156.49, 152.92, 152.62, 148.62, 146.74, 129.40, 127.39, 126.46 (d, J = 12 Hz), 119.23 (d, J = 23.2 Hz), 117.4 (d, J = 8.3 Hz), 109.96, 108.08, 102.23, 56.09.