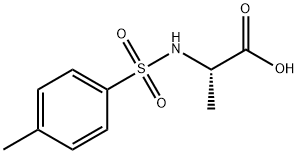
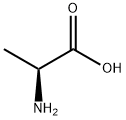
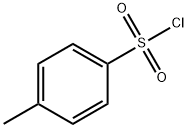
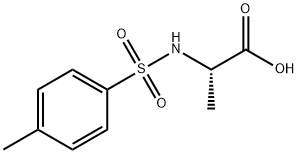
GENERAL STEPS: The synthesis was carried out using the N-toluene sulfonylation method reported in the literature. In a round-bottomed flask, 2.4 equivalents of Na2CO3 was dissolved in water at 70 °C (concentration 0.66 mol/L). Subsequently, 1.0 equiv. of L-alanine was added, followed by the slow addition of 1.4 equiv. of p-toluenesulfonyl chloride. The resulting suspension was stirred and reacted at 70 °C for 40 min, followed by warming to 85 °C and continued stirring for 5 min. Upon completion of the reaction, the reaction mixture was filtered directly and the solids were washed with hot water at 85°C. The filtrate was cooled to room temperature and acidified with 6 M aqueous HCl to pH=1. The precipitated solid product was collected by filtration and dried under vacuum. The resulting 2-(4-methylphenylsulfonamido)propionic acid was used for subsequent experiments without further purification.
[1] RSC Advances, 2015, vol. 5, # 24, p. 18751 - 18760
[2] European Journal of Organic Chemistry, 2018, vol. 2018, # 29, p. 4006 - 4012
[3] Organic and Biomolecular Chemistry, 2013, vol. 11, # 21, p. 3451 - 3460
[4] Zeitschrift fur Naturforschung - Section B Journal of Chemical Sciences, 2000, vol. 55, # 2, p. 203 - 207
[5] Journal of Organic Chemistry, 2003, vol. 68, # 17, p. 6632 - 6638