Chemical Properties
| Melting point | 224-229 °C (dec.) |
| Density | 1.07607 g/cm3(Temp: 25.00 °C) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Powder |
| color | Off-white to yellow |
| pka | 7.6(at 25℃) |
| PH | 7.0-8.2 |
| PH Range | 7.0 - 8.2 |
| Water Solubility | soluble |
| Merck | 13,9148 |
| BRN | 2113949 |
| Major Application | diagnostic assay manufacturing |
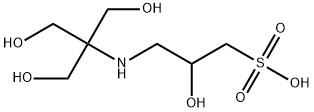
| InChI | InChI=1S/C7H17NO7S/c9-3-7(4-10,5-11)8-1-6(12)2-16(13,14)15/h6,8-12H,1-5H2,(H,13,14,15) |
| InChIKey | RZQXOGQSPBYUKH-UHFFFAOYSA-N |
| SMILES | C(S(O)(=O)=O)C(O)CNC(CO)(CO)CO |
| CAS DataBase Reference | 68399-81-5(CAS DataBase Reference) |
| EPA Substance Registry System | 1-Propanesulfonic acid, 2-hydroxy-3-[[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]amino]- (68399-81-5) |
Safety Information
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36 |
| WGK Germany | 3 |
| TSCA | TSCA listed |
| HazardClass | IRRITANT |
| HS Code | 29221990 |
| Storage Class | 11 - Combustible Solids |
| Hazard Classifications | Eye Irrit. 2 Skin Irrit. 2 STOT SE 3 |