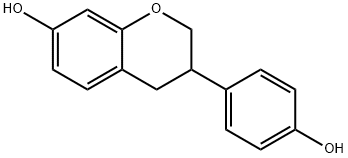
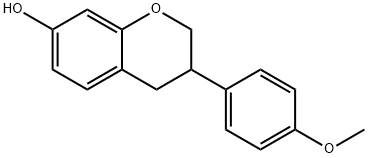
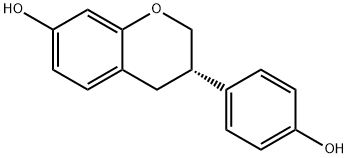
A mixture of compound 3a (0.20 g, 0.78 mmol), n-Bu4NI (1.01 g, 2.73 mmol) and anhydrous CH2Cl2 (5 mL) was stirred and cooled to -78 °C under nitrogen protection. Subsequently, a CH2Cl2 solution of BCl3 (1 M, 2.73 mL, 2.73 mmol) was added dropwise to the cooled mixture. The reaction mixture was stirred and slowly warmed to 0 °C over a period of 2 hours. Upon completion of the reaction, it was carefully quenched with ice water (10 mL), stirring was continued for 20 min, and then neutralized with saturated NaHCO3 solution (10 mL). After vacuum concentration, the residue was extracted with EtOAc (10 mL x 0.5). The organic layers were combined and dried with anhydrous MgSO4. After filtration, the filtrate was concentrated under vacuum and purified by silica gel column chromatography (ethyl acetate/hexane=1:3) to give colorless crystals of estragole 3b (0.18 g, 95% yield). The properties of estragole were as follows: melting point 162-164 °C (literature value 158 °C); thin-layer chromatography Rf value 0.24 (ethyl acetate/hexane=1:3); infrared spectra (KBr) νmax: 3231, 2897, 1598, 1510, 1470, 1366, 1252, 1158, 1119, 826 cm-1; 1H-NMR ( 400 MHz, DMSO-d6) δ: 2.80 (m, 2H, H-3 and H-4a), 3.01 (m, 1H, H-4b), 3.89 (t, J=10.4 Hz, 1H, H-2a), 4.14 (d, J=10.4 Hz, 1H, H-2b), 6.20 (d, J=2.4 Hz, 1H, H-8), 6.29 (dd, J=8.4, 2.4 Hz, 1H, H-6), 6.72 (d, J=8.4 Hz, 2H, H-3', H-5'), 6.86 (d, J=8.4 Hz, 1H, H-5), 7.10 (d, J=8.4 Hz, 2H, H-2', H-6'), 9.18 (s, 1H, OH), 9.29 (s, 1H, OH); 13C-NMR (100 MHz, DMSO-d6) δ: 31.3, 37.2, 70.3, 102.5, 108.0, 112.6, 115.3, 128.3, 130.1, 131.7, 154.5, 156.2, 156.5; electron bombardment mass spectrometry (70 eV) m/z (relative intensities, %): 242 (M+, 58), 135 (25), 134 (40), 123 (88), 120 (100), 107 (39), 91 (37). Calculated elemental analysis (C15H14O3): C 74.36, H 5.82; measured values: C 74.13, H 5.90.