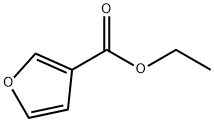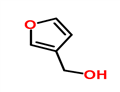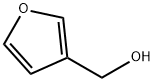
3-FURANMETHANOL
- Product Name3-FURANMETHANOL
- CAS4412-91-3
- MFC5H6O2
- MW98.1
- EINECS224-570-7
- MOL File4412-91-3.mol
Chemical Properties
| Boiling point | 79-80 °C17 mm Hg(lit.) |
| Density | 1.139 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point | 101 °F |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| pka | 14.49±0.10(Predicted) |
| form | Liquid |
| color | Clear colorless to slightly yellow |
| BRN | 106456 |
| InChI | InChI=1S/C5H6O2/c6-3-5-1-2-7-4-5/h1-2,4,6H,3H2 |
| InChIKey | STJIISDMSMJQQK-UHFFFAOYSA-N |
| SMILES | O1C=CC(CO)=C1 |
| LogP | 0.300 |
| CAS DataBase Reference | 4412-91-3(CAS DataBase Reference) |
Safety Information
| Hazard Codes | Xi,F |
| Risk Statements | 10-36/37/38 |
| Safety Statements | 16-26-36/37/39-36/37 |
| RIDADR | UN 1987 3/PG 3 |
| WGK Germany | 3 |
| HazardClass | 3.2 |
| PackingGroup | III |
| HS Code | 29321900 |
| Storage Class | 3 - Flammable liquids |
| Hazard Classifications | Eye Irrit. 2 Flam. Liq. 3 Skin Irrit. 2 STOT SE 3 |