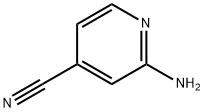
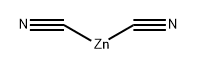
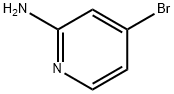
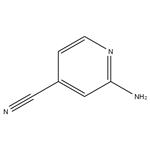
Step 5: A mixture of 4-bromopyridin-2-amine (4.9 g, 28.5 mmol), zinc cyanide (5.0 g, 42.5 mmol), tris(dibenzylideneacetone)dipalladium (1.3 g, 1.4 mmol), and 1,1'-bis(diphenylphosphino)ferrocene (1.6 g, 2.8 mmol) was dissolved in N,N-dimethylformamide (150 mL). The reaction mixture was stirred at 100 °C for 1.5 h under nitrogen protection. After completion of the reaction, the mixture was cooled to room temperature and diluted with water (500 mL). The mixture was extracted with ethyl acetate (300 mL x 3). The organic layers were combined, washed with saturated saline, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The residue was purified by silica gel column chromatography with the eluent petroleum ether/ethyl acetate (10:1 to 2:1) to afford 2-amino-4-cyanopyridine as a light yellow solid (2.7 g, 80% yield).1H NMR (300 MHz, CDCl3) δ 8.19 (d, J = 5.1 Hz, 1H), 6.82 (d, J = 4.8 Hz, 1H) 6.69 (d, J = 0.9 Hz, 1H), 4.72 (br s, 2H).LCMS (ESI) m/z 120 (M + H)+.