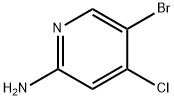
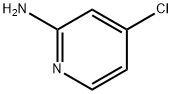
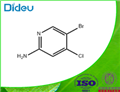
The general procedure for the synthesis of 2-amino-4-chloro-5-bromopyridine from 2-amino-4-chloropyridine was as follows: 4-chloropyridin-2-amine (8 g, 62.2 mmol) was dissolved in acetonitrile (600 mL) at room temperature, and N-bromosuccinimide (NBS, 11.08 g, 62.2 mmol) was added in batches under stirring. The reaction mixture was stirred continuously for 14 hours at room temperature. After completion of the reaction, the solvent was removed by concentration under reduced pressure. The residue was redissolved in a solvent mixture of ethyl acetate and water. The organic phase was extracted with ethyl acetate (3 x 50 mL) and the combined organic layers were washed sequentially with water (100 mL) and brine (100 mL) and dried over anhydrous sodium sulfate. Concentration of the organic phase under reduced pressure afforded 5-bromo-4-chloropyridin-2-amine as a yellow solid (13 g, 99% yield), which could be used for subsequent reactions without further purification. The product was confirmed by LC-MS (ESI), m/z 207.0 [(M + H)+, calculated value 206.9 for C5H5BrClN2]; LC/MS retention time (Method B): tR = 0.8 min; 1H NMR (400 MHz, CDCl3) δ 8.17 (s, 1H), 6.63 (s, 1H), 4.59 (s, 2H) .