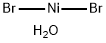
NICKEL(II) BROMIDE TRIHYDRATE
- Product NameNICKEL(II) BROMIDE TRIHYDRATE
- CAS7789-49-3
- MFBr2H2NiO
- MW236.52
- EINECS677-463-6
- MOL File7789-49-3.mol
Chemical Properties
| Melting point | 963 °C(lit.) |
| Density | 5.098 g/mL at 25 °C(lit.) |
| form | powder |
| Water Solubility | Soluble in water, alcohol |
| Sensitive | Hygroscopic |
| Merck | 14,6502 |
| Exposure limits | ACGIH: TWA 0.1 mg/m3 NIOSH: IDLH 10 mg/m3; TWA 0.015 mg/m3 |
| Major Application | battery precursors catalysts material synthesis precursor |
| InChI | 1S/2BrH.Ni.3H2O/h2*1H;;3*1H2/q;;+2;;;/p-2 |
| InChIKey | UQPSGBZICXWIAG-UHFFFAOYSA-L |
| SMILES | O.O.O.Br[Ni]Br |
| CAS DataBase Reference | 7789-49-3(CAS DataBase Reference) |
Safety Information
| Hazard Codes | T,N |
| Risk Statements | 45-22-43-50/53 |
| Safety Statements | 53-36/37-45-60-61 |
| RIDADR | UN 3077 9/PG 3 |
| WGK Germany | 3 |
| TSCA | Yes |
| HazardClass | 6.1 |
| PackingGroup | III |
| Storage Class | 6.1D - Non-combustible acute toxic Cat.3 toxic hazardous materials or hazardous materials causing chronic effects |
| Hazard Classifications | Aquatic Acute 1 Aquatic Chronic 1 Carc. 1A Inhalation Muta. 2 Repr. 1B Resp. Sens. 1 Skin Sens. 1 STOT RE 1 Inhalation |


