7-Chloro-1,2,3,4-tetrahydrobenzo[b]azepin-5-one is an intermediate in the synthesis of Tolvaptan (T536650) (OPC-41061), an orally active nonpeptide arginine vasopressin V2 receptor antagonist
The synthetic method of 7-Chloro-1,2,3,4-tetrahydrobenzo(b)azepin-5-one comprises the following steps: carrying out an acylation reaction between 4-chloroaniline and succinic anhydride to obtain 4-(4-chloroaniline)-4-oxobutyric acid; carrying out an intramolecular Friedel-Craft reaction on 4-(4-chloroaniline)-4-oxobutyric acid to obtain 7-chloro-3,4-tetrahydrobenzo[b]azepine-2,5-one; reacting 7-chloro-3,4-tetrahydrobenzo[b]azepine-2,5-one with ethylene glycol to obtain 7-chloro-3,4-tetrahydrobenzo[b]azepine-2-one-5-glycol ketal; reducing 7-chloro-3,4-tetrahydrobenzo[b]azepine-2-one-5-glycol ketal, and carrying out de-ketalation under an acidic condition to obtain 7-Chloro-1,2,3,4-tetrahydrobenzo(b)azepin-5-one.
[1] Patent: WO2007/26971, 2007, A2. Location in patent: Page/Page column 28
[2] Bioorganic and Medicinal Chemistry, 1999, vol. 7, # 8, p. 1743 - 1754
[3] Tetrahedron Asymmetry, 2010, vol. 21, # 19, p. 2390 - 2393
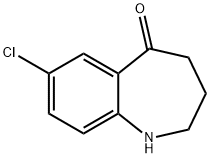

![7-chloro-1,2,3,4-tetrahydro-benzo[b]azepin-5-one pictures](/ProductImageEN1/2024-07/Small/1c87e63f-70fb-4f6f-b866-41d00a3f255d.jpg)