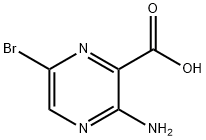
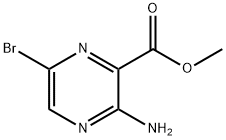
Step 2: Methyl 3-amino-6-bromopyrazine-2-carboxylate (5.11 g, 22.02 mmol) and lithium hydroxide (2.637 g, 110.1 mmol) were dissolved in a mixed solvent of methanol (20 mL) and water (20 mL), heated to 90 °C, and the reaction was carried out for 2 hours. After completion of the reaction, the reaction mixture was cooled, neutralized with hydrochloric acid to pH neutral, and the precipitated solid was collected by filtration. The resulting 3-amino-6-bromopyrazine-2-carboxylic acid was used directly in the next step of the reaction without further purification (4.80 g, 99% yield).
[1] Patent: WO2011/143419, 2011, A1. Location in patent: Page/Page column 44-45
[2] Bioorganic and Medicinal Chemistry Letters, 2012, vol. 22, # 8, p. 2784 - 2788
[3] Journal of Medicinal Chemistry, 2011, vol. 54, # 7, p. 2320 - 2330
[4] Patent: WO2017/123588, 2017, A1. Location in patent: Paragraph 0031; 0032; 0033
[5] Patent: WO2010/54398, 2010, A1. Location in patent: Page/Page column 90