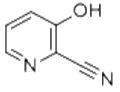
Example 3: 5.0 g of 1N hydrochloric acid and 1.0 g (6.31 mmol) of α-amino-2-furan acetonitrile hydrochloride were added to a three-neck flask at room temperature. The reaction mixture was cooled to 0 °C and 360 μL of bromine (6.94 mmol) was slowly added dropwise, followed by stirring at 0 °C for 1 hour. The reaction mixture was gradually warmed to room temperature and stirring was continued for 2 hours. After that, 32 μL of bromine was added to the reaction system and stirring was continued for 2 hours. Again, 32 μL of bromine was added and stirred for 1 hour. Upon completion of the reaction, 199 mg of sodium thiosulfate was added to the mixture to quench the reaction, followed by adjusting the pH with 40% NaOH aqueous solution to 3.0. The reaction mixture was filtered to afford a product containing 0.47 g of 2-cyano-3-hydroxypyridine. The yield of 2-cyano-3-hydroxypyridine was 62% based on the amount of α-amino-2-furylacetonitrile hydrochloride.
[1] Patent: TW2016/510, 2016, A. Location in patent: Page/Page column 14
[2] Patent: WO2017/127794, 2017, A1
[3] Patent: WO2017/127791, 2017, A1
[4] Patent: TW2018/27404, 2018, A