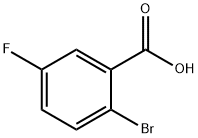
2-Bromo-5-fluorobenzoic acid, a common organic synthesis intermediate, is primarily used to synthesize target molecular structures by utilizing the bromine and fluorine atoms on the benzene ring. For example, the bromine atom can undergo Suzuki coupling to introduce an aromatic ring or alkyl chain; or it can be converted into boric acid or borate esters, leveraging the versatility of borate esters to synthesize complex molecules. The fluorine atom can also undergo a series of transformations, including aromatic nucleophilic substitution reactions. Finally, the carboxyl group on the benzene ring can be readily converted into amides, esters, viboramides, etc.
white to light yellow crystal powder
2-Bromo-5-fluorobenzoic acid is synthesized from 5-(2′-bromophenoxy)benzothiazole and 2,3,4,5,6-pentafluorobenzoyl chloride in three steps. The synthesis involves bromination of benzothiazole with NBS followed by reaction with 4-fluoroaniline to give the intermediate which is then reacted with pentafluorobenzoyl chloride.
[1] Patent: WO2005/56544, 2005, A1. Location in patent: Page/Page column 46