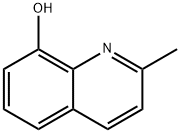
beige to brown crystalline powder. Soluble in hot alcohol, ether and benzene, insoluble in water. Can volatilize with water vapor and sublimate at 100℃. With phenol odor.
8-Hydroxyquinaldine is mainly used as metal ion extractant and metal ion determination, also used in the synthesis of dyes and pigments and the preparation of pharmaceutical intermediates.
8-hydroxyquinaldine is obtained from the reaction of 2-aminophenol with crotonaldehyde. Mix 2-aminophenol and 2-Nitrophenol homogeneously and add hydrochloric acid. Add crotonaldehyde under stirring. Heat for 6h and leave overnight. Distill the reacted o-nitrophenol by water distillation, and add sodium hydroxide solution to the residual solution to make it weakly basic. Then add powdered carbonyls for saturation, and distill 8-Hydroxyquinaldine by water distillation, and then distill the crude product under reduced pressure and recrystallize it with ethanol to obtain 8-hydroxyquinaldine.
ChEBI: 2-Methylquinolin-8-ol is a hydroxyquinoline.
2-Methyl-8-quinolinol is a methyl substituted quinolinol derivative that shows fungicidal property. It can also undergo complexation with transition metal complexes.
Crystallise the quinoline from EtOH or aqueous EtOH. Its solubility at 20o in H2O is 0.366g/L, and in CHCl3 it is 466g/L. It complexes with many metals. [Beilstein 21 H 106, 21 III/IV 2132.]