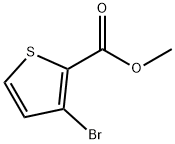
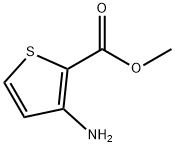
The general procedure for the synthesis of methyl 3-bromothiophene-2-carboxylate from methyl 3-amino-2-thiophenecarboxylate was as follows: methyl 3-amino-2-thiophenecarboxylate (100 g, 0.6369 mol) was suspended in hydrobromic acid (220 mL) and stirred at room temperature for 15 min. Subsequently, the mixture was cooled to 0-5 °C and a solution of sodium nitrite (46.0 g, 0.666 mol) in water (100 mL) was slowly added dropwise below 5 °C. After the dropwise addition, stirring was continued for 1 hour. The above mixture was then added to a solution of copper(I) bromide (96.0 g, 0.6692 mol) in hydrobromic acid (260 mL) at room temperature. The reaction system was warmed to 60-65 °C and the reaction was stirred for 2 hours. Upon completion of the reaction, the reaction mixture was diluted with 1200 mL of water at a controlled temperature of 25-30 °C and extracted with two portions of 600 mL of ethyl acetate. The organic phases were combined, washed twice successively with 600 mL of water and dried over anhydrous sodium sulfate. Ethyl acetate was removed by distillation under reduced pressure (60 °C) to give a yellow solid product of 129.0 g in 91.6% yield, melting point 47-48 °C, and HPLC purity of 96%. The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ 3.90 (s, 3H, OCH3), 7.09 (d, 1H), 7.46 (d, 1H); 13C NMR (400 MHz, CDCl3): δ 52.10, 116.96, 127.12, 130.61, 133.63, 161.0; mass spectrum (MS): m/z 222.8 [M+1].