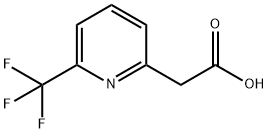
Tert-butyl 6-(trifluoromethyl)-2-pyridylacetate (698 mg, 2.67 mmol) was used as a raw material and dissolved in dichloromethane (10 mL). Triethylsilane (1.067 mL, 6.68 mmol) and trifluoroacetic acid (2.68 mL, 34.7 mmol) were added dropwise via syringe at room temperature. The reaction mixture was stirred at room temperature overnight. After confirming good conversion of the reaction by LC-MS analysis, the reaction solution was concentrated to give a yellow oily substance. Diethyl ether (5 mL) was added to the oily material, no precipitate formation was observed, and the solution was subsequently concentrated to afford 2-(6-(trifluoromethyl)pyridin-2-yl)acetic acid (535 mg, 2.61 mmol, 98% yield) as a yellow oil, which solidified to a yellow solid upon standing.LC-MS (ES) m/z = 206 [M + H]+.1H NMR (400 MHz , DMSO-d6) δ ppm 3.89 (s, 2H), 7.70 (d, J = 7.83 Hz, 1H), 7.81 (d, J = 7.58 Hz, 1H), 7.97-8.16 (m, 1H), 12.26-12.88 (br.s., 1H).