Antibacterial.This compound is a contaminant of emerging concern (CECs).
Sulfonamide antibacterial.
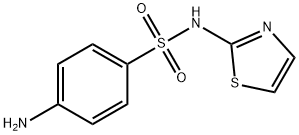
ChEBI: A 1,3-thiazole compound having a 4-aminobenzenesulfonamido group at the 2-position.
116 parts 4-acetamidobenzolsufonyl chloride (prepared from acetanilide and
chlorosulfonic acid) was mixed with 100 parts 2-aminothiasole in 1000 parts
water by cooling and stirred for some hours. The bis-amide obtained was
filtered off and re-crystallized from 50% ethanol to give bis-(pacetylaminobenzolsulfo)-
2-aminothiazol with MP: 129°C.
10 parts above bis-amide was heated with 10% sodium hydroxide solution for
0.5 hour on water bath. On cooling and filtration the alkaline solution was
acidified with glacial acetic acid. The amide obtained was cleared by recrystallized
from water to give N1-2-thiazolylsulfanilamide; MP: 202°-203°C.
Argazol;Bucosol;Chemiovis;Coryza;Crionil;Csp 500;Csp-250;Edifeno;Femakzem;Formotablin antidiarreico;Gyne-sulf;Gyn-sulf;Neosutrin;Polvos wilfe;Pomada wilfe;Prothiazol;Septex cream no. 2;Streptacillin;Sulfa-orzon;Sulfazol;Sulfhatose;Sulfintestin;Sulfopyrol;Sulfour;Sulfzol;Sulnac;Sulphatriad;Tampovagan pss;Thiadyl;Thiuramide;Tiadyl;Trimeto;Trisulpha;Trysul;Tylasul;Ufa 902-duo;Vetoprim mi;Wintrazol.
World Health Organization (WHO)
Sulfathiazole, a sulfonamide anti-infective agent, was introduced
more than 25 years ago for the treatment of bacterial infections. The importance of
sulfonamides has subsequently decreased as a result of increasing bacterial
resistance and their replacement by antibiotics which are generally more active
and less toxic. The sulfonamides are known to cause serious adverse effects such
as renal toxicity, sometimes fatal exfoliative dermatitis and erythema multiforma
and dangerous adverse reactions affecting blood formation such as
agranulocytosis and haemolytic or aplastic anaemia. Although preparations remain
available, use of the drug has been discontinued in many countries.
White crystalline powder. Is dimorphous: form I is consists of prismatic rods and form II of six-sided plates and prisms. Insoluble in water and soluble in dil aqueous acid and aqueous base.
May be sensitive to heat, air and light during long-term storage . Insoluble in water.
Sulfathiazole is an amino acid.
Flash point data for Sulfathiazole are not available, but Sulfathiazole is probably combustible.
Pharmaceutical Applications
2-Sulfanilamidothiazole. A short-acting compound (half-life
c. 4 h) with relatively high activity. Protein binding is c. 75%.
Its use has declined because of a high incidence of side effects.
It is one of the constituents of triple sulfonamide mixtures, of
which local preparations are still available.
Two compounds, phthalylsulfathiazole (sulfathalidine) and
succinylsulfathiazole (sulfasuxidine) owe their activity to the
slow liberation of sulfathiazole in the bowel. They are poorly
soluble and very little is absorbed after oral administration.
They were formerly used in the treatment of intestinal infections
and in bowel preparation before surgery. They are available
in multi-ingredient preparations in some countries.
Sulfonamide antibiotic that blocks the synthesis of dihydrofolic acid by inhibiting the enzyme dihydropteroate synthase.Mode of Action: Inhibits folic acid synthesis in prokaryotes.Anti-microbial Spectrum: Gram positive, Gram negative, ChlamydiaMode of Resistance: Alteration of dihydropteroate synthase or alternative pathway for folic acid synthesis.
Human poison by
unspecified route. Experimental poison by
intraperitoneal route, Moderately toxic by
intravenous, subcutaneous, and parenteral
routes. Mildly toxic by ingestion. Human
systemic effects by unspecified route:
conjunctiva irritation, tubule changes, and
allergic skin dermatitis. Experimental
reproductive effects. Questionable
carcinogen with experimental tumorigenic
data, Mutation data reported. When heated
to decomposition it emits very toxic fumes
of NOx and SOx.