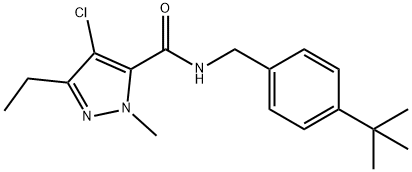
Tebufenpyrad
- Product NameTebufenpyrad
- CAS119168-77-3
- MFC18H24ClN3O
- MW333.86
- EINECS242-070-7
- MOL File119168-77-3.mol
Chemical Properties
| Melting point | 61-62° |
| Boiling point | 468.4±45.0 °C(Predicted) |
| Density | 1.1639 (rough estimate) |
| refractive index | 1.5790 (estimate) |
| storage temp. | 0-6°C |
| solubility | Benzene (Slightly), DMSO (Slightly), Methanol (Slightly) |
| pka | 13.19±0.46(Predicted) |
| form | solid |
| BRN | 8636471 |
| InChI | InChI=1S/C18H24ClN3O/c1-6-14-15(19)16(22(5)21-14)17(23)20-11-12-7-9-13(10-8-12)18(2,3)4/h7-10H,6,11H2,1-5H3,(H,20,23) |
| InChIKey | ZZYSLNWGKKDOML-UHFFFAOYSA-N |
| SMILES | N1(C)C(C(NCC2=CC=C(C(C)(C)C)C=C2)=O)=C(Cl)C(CC)=N1 |
| LogP | 4.610 |
| CAS DataBase Reference | 119168-77-3(CAS DataBase Reference) |
| EPA Substance Registry System | Tebufenpyrad (119168-77-3) |
Safety Information
| Hazard Codes | Xn |
| Risk Statements | 22 |
| Safety Statements | 36 |
| RIDADR | 2588 |
| WGK Germany | 3 |
| RTECS | UQ6276400 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| HS Code | 29331990 |
| Storage Class | 6.1C - Combustible acute toxic Cat.3 toxic compounds or compounds which causing chronic effects |
| Hazard Classifications | Acute Tox. 3 Oral Acute Tox. 4 Inhalation Aquatic Acute 1 Aquatic Chronic 1 Skin Sens. 1B STOT RE 2 |
| Hazardous Substances Data | 119168-77-3(Hazardous Substances Data) |
| Toxicity | LD50 in male, female rats (mg/kg): 595, 997 orally; >2000, >2000 dermally; LC50 in male rats (mg/m3): 2660 by inhalation (Inoue, Fukuchi) |


