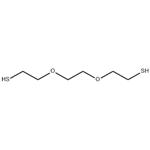
3,6-DIOXA-1,8-OCTANEDITHIOL
- Product Name3,6-DIOXA-1,8-OCTANEDITHIOL
- CAS14970-87-7
- MFC6H14O2S2
- MW182.3
- EINECS239-044-2
- MOL File14970-87-7.mol
Chemical Properties
| Boiling point | 225 °C(lit.) |
| Density | 1.12 g/mL at 25 °C(lit.) |
| vapor pressure | 0.39Pa at 20℃ |
| refractive index | n |
| Flash point | 132 °C |
| storage temp. | RT, stored under nitrogen |
| solubility | Slightly soluble in water |
| form | clear liquid |
| pka | 9.34±0.10(Predicted) |
| color | Colorless to Light yellow to Light orange |
| Water Solubility | 11.4g/L at 19.8℃ |
| BRN | 1901671 |
| InChI | InChI=1S/C6H14O2S2/c9-5-3-7-1-2-8-4-6-10/h9-10H,1-6H2 |
| InChIKey | HCZMHWVFVZAHCR-UHFFFAOYSA-N |
| SMILES | C(OCCS)COCCS |
| LogP | 1.6 at 55℃ |
| CAS DataBase Reference | 14970-87-7(CAS DataBase Reference) |
| EPA Substance Registry System | Ethanethiol, 2,2'-[1,2-ethanediylbis(oxy)]bis- (14970-87-7) |
Safety Information
| Hazard Codes | T,N,Xn |
| Risk Statements | 22-23-36/37/38-51/53-20/22 |
| Safety Statements | 26-45-61 |
| RIDADR | UN 2810 6.1/PG 3 |
| WGK Germany | 2 |
| TSCA | TSCA listed |
| HS Code | 29309090 |
| Storage Class | 6.1C - Combustible acute toxic Cat.3 toxic compounds or compounds which causing chronic effects |
| Hazard Classifications | Acute Tox. 3 Oral Acute Tox. 4 Inhalation Aquatic Acute 1 Aquatic Chronic 1 |