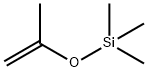
ISOPROPENYLOXYTRIMETHYLSILANE
- Product NameISOPROPENYLOXYTRIMETHYLSILANE
- CAS1833-53-0
- MFC6H14OSi
- MW130.26
- EINECS217-393-1
- MOL File1833-53-0.mol
Chemical Properties
| Melting point | 40-45 °C |
| Boiling point | 93-95 °C |
| Density | 0.78 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point | 45 °F |
| storage temp. | -20°C |
| form | clear liquid |
| color | Colorless to Light orange to Yellow |
| Specific Gravity | 0.786 |
| Hydrolytic Sensitivity | 7: reacts slowly with moisture/water |
| BRN | 1849023 |
| InChI | 1S/C6H14OSi/c1-6(2)7-8(3,4)5/h1H2,2-5H3 |
| InChIKey | UAIFZYSPVVBOPN-UHFFFAOYSA-N |
| SMILES | CC(=C)O[Si](C)(C)C |
| CAS DataBase Reference | 1833-53-0(CAS DataBase Reference) |
| NIST Chemistry Reference | CH2=(CH3)OSi(CH3)3(1833-53-0) |
| EPA Substance Registry System | Silane, trimethyl[(1-methylethenyl)oxy]- (1833-53-0) |
Safety Information
| Hazard Codes | F,Xi |
| Risk Statements | 11-36/37/38 |
| Safety Statements | 16-26-36-37/39 |
| RIDADR | UN 1993 3/PG 2 |
| WGK Germany | 3 |
| F | 10-21 |
| TSCA | TSCA listed |
| HazardClass | 3.1 |
| PackingGroup | II |
| HS Code | 2931900090 |
| Storage Class | 3 - Flammable liquids |
| Hazard Classifications | Eye Irrit. 2 Flam. Liq. 2 Skin Irrit. 2 STOT SE 3 |


