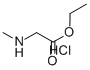
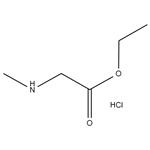
Thiophenecarbonyl chloride (65 mL, 900 mmol) was added dropwise to a solution of sarcosine (20.0 g, 224 mmol) in ethanol (250 mL) cooled in an ice-water bath under stirring conditions, and the reaction temperature was maintained at about -10°C. After the dropwise addition was completed, the reaction mixture was gently heated at 55°C overnight until the solution became clear. Upon completion of the reaction, the solvent and residual thionyl chloride were removed by distillation under reduced pressure. The resulting solid residue was washed with ether (3 x 50 mL) and subsequently dried well under vacuum to afford ethyl sarcosinate hydrochloride (33.5 g, 218 mmol) in white powder form in 97% yield. The product could be used in subsequent steps without further purification. The product was characterized as follows: melting point 126°C (literature value 125-127°C); IR (ATR) ν cm1 2970-2440, 1742, 1229; 1H NMR (CDCl, 400 MHz) δ 9.64 (br.s, 2H, NH), 4.24 (q, 2H, 3J = 7.1 Hz, CHCH), 3.84 (t, 2H, 3J = 7.1 Hz, CHCH), 4.24 (q, 2H, 3J = 7.1 Hz, CH 3.84 (t, 2H, 3J = 5.7 Hz, NHCH), 2.80 (t, 3H, 3J = 5.2 Hz, NHCH), 1.26 (t, 3H, 3J = 7.1 Hz, CHCH); 13C NMR (CDCl, 101 MHz) δ 166.18 (CO), 62.62 (CHCH), 48.94 (NHCH), 33.34 (NHCH), 14.03 (CHCH).