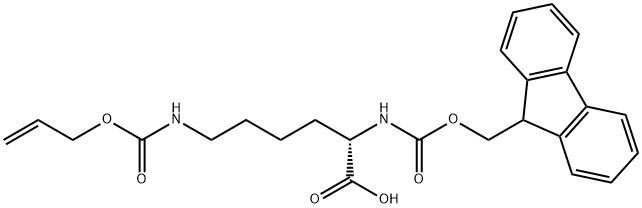
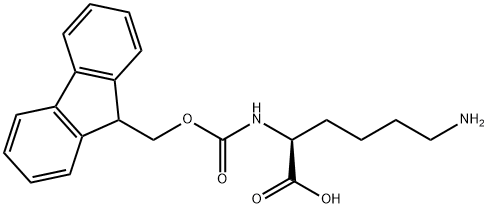
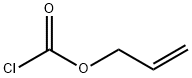
The general procedure for the synthesis of N-[(9H-fluoren-9-ylmethoxy)carbonyl]-N'-[(2-propenyloxy)carbonyl]-L-lysine from (S)-2-((9H-fluoren-9-ylmethoxy)methoxy)carbonyl)amino)-6-aminohexanoic acid and allyl chloroformate was as follows:
Example L1c: 25 g (61.7 mmol) of (S)-6-amino-2-(9H-fluoren-9-ylmethoxycarbonylamino)-hexanoic acid was dissolved in 275 mL of dioxane and mixed with 25 mL of 25% potassium carbonate solution, followed by the addition of 6.55 mL of allyl chloroformate. The reaction mixture was stirred at 23°C for 20 hours. Upon completion of the reaction, the reaction solution was diluted with water and extracted with methyl tert-butyl ether. The aqueous phase was separated, acidified with 2N hydrochloric acid and extracted with dichloromethane several times. The dichloromethane phase was combined and dried with sodium sulfate. After filtration, the solvent was removed under reduced pressure to afford 26.3 g (58.1 mmol, 94%) of N-[(9H-fluoren-9-methoxy)carbonyl]-N'-[(2-propenyloxy)carbonyl]-L-lysine, and the product could be used for subsequent reactions without further purification.