Filgotinib (GLPG0634) is a selective JAK1 inhibitor with IC50 of 10 nM, 28 nM, 810 nM, and 116 nM for JAK1, JAK2, JAK3, and TYK2, respectively.
In cell lines, GLPG0634 inhibits IL-2- and IL-4-induced JAK1/JAK3/γc signaling and IFN-αB2-induced JAK1/TYK2 type II receptor signaling with IC50 ranged from 150 to 760 nM. GLPG0634 shows higher selectivity for JAK/STAT signaling involving JAK1 than JAK2 kinase in a cellular context. Besides, GLPG0634 also inhibits the differentiation of Th1, Th2, and Th17 cells.
Following oral administration, the absolute bioavailability is moderate in rats (45%) and high in mice (~100%). Filgotinib (30 mg/kg daily (Rats); 50 mg/kg twice daily (Mice)) dose-dependently reduces inflammation, cartilage, and bone degradation in the CIA model in rats and mice. Filgotinib (GLPG0634) in DSS-treated mice demonstrates that inhibition of JAK1 is sufficient for achieving strong efficacy in pre-clinical mouse model, correlated to the inhibition of STAT3 phosphorylation in the inflamed colon.
Filgotinib is a JAK1 inhibitor (IC50 = 10 nM). It is selective for JAK1 over JAK3 (IC50 = 810 nM) but also inhibits JAK2 and tyrosine kinase 2 (Tyk2; IC50s = 28 and 116 nM, respectively), as well as Abl, FLT1, -3, and -4, FMS, Mer, and TBK1 activity by greater than 35% in a panel of 177 tyrosine kinases at 1 μM. Filgotinib inhibits IL-6-induced phosphorylation of STAT1 in CD4+ T cells with an IC50 value of 629 nM in isolated human whole blood. It reduces hind paw macrophage and T cell infiltration and bone erosion in a rat model of collagen-induced arthritis when administered at doses ranging from 0.1 to 30 mg/kg per day for 15 days.
Class: non-receptor tyrosine kinase
Treatment: ulcerative colitis, rheumatoid arthritis
Elimination half-life = 6–11 h
Protein binding = 32%
GLPG-0634 (Filgotinib) is a potent, selective JAK1 inhibitor. Used as an anti-inflammatory agent, it reduces levels of inflammatory cytokines in the bones and tissue of mammals.
Filgotinib acts on the JAK-STAT pathway by selectively inhibiting JAK1 phosphorylation and preventing STAT activation, which ultimately results in reduced proinflammatory cytokine signaling.
- feeling sick (nausea)
- upper respiratory tract infection
- urinary tract infection.
- dizziness.
The synthesis of Filgotinib is as follows:
With cyclopropanyl chloride in an amidated reaction in a system consisting of N-methylmorpholine and 1,4-dioxane,The amidation reaction time was 4 h,The amidation reaction temperature was 50 ° C,The molar ratio of the intermediate (III), cyclopropanyl chloride, N-methylmorpholine and 1,4-dioxane was 1: 2.8: 2.5:TLC plate to determine the reaction is completed, cooled to room temperature,Adding methylene chloride and water, separating the organic phase with water,Then washed with brine, dried over magnesium sulfate,Evaporated to dryness and the residue was purified over a silica gel column [elution solvent: ethyl acetate / n-hexane (3: 7 v / v)To obtain a solid yellowish solid,That is, Filgotinib, yield 91.2%.

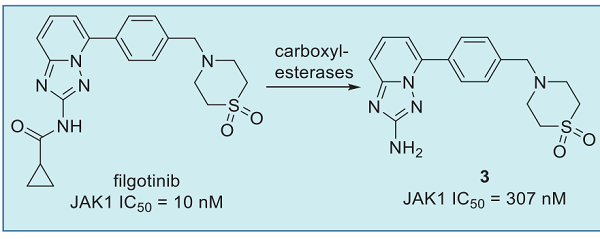
Filgotinib has favorable metabolic stability in vitro (65%, 45%, and 87% of compound remaining in rat, dog, and human liver microsomes, respectively, after 60 min incubation; and a half-life of more than 200 min in hepatocytes for all species). Filgotinib is a substrate of P-gp, as revealed by an efflux ratio of 16 from the Caco-2 assay. In spite of the high efflux ratio, it still showed 45% and 67% oral bioavailability in rats and dogs, respectively, presumably due to its good solubility (0.18 mg/mL in water), high membrane permeability, and good metabolic stability. It exhibited low plasma protein bindings across species (48% in rats, 29% in dogs, and 32% in humans). The elimination half-lives of filgotinib were 6 and 11 hours in the Japanese and Caucasian volunteers, respectively. In both trials, the active metabolite 3 showed plasma concentrations far beyond those of filgotinib, with half-life values ranging from 17 to 20 hours, which supports once-a-day oral administration. The active metabolite 3 was formed by hydrolysis of the amide functionality to release the free amine. This metabolite is less potent than filgotinib but still maintains good JAK1 selectivity. This metabolite is believed to contribute to the overall therapeutic effects along with the parent drug.
[1] CHRISTEL J. MENET*. Triazolopyridines as Selective JAK1 Inhibitors: From Hit Identification to GLPG0634[J]. Journal of Medicinal Chemistry, 2014, 57 22: 9323-9342. DOI:
10.1021/jm501262q[2] LUC VAN ROMPAEY. Preclinical characterization of GLPG0634, a selective inhibitor of JAK1, for the treatment of inflammatory diseases.[J]. Journal of immunology, 2013, 191 7: 3568-3577. DOI:
10.4049/jimmunol.1201348[3] A KAVANAUGH. Filgotinib (GLPG0634/GS-6034), an oral selective JAK1 inhibitor, is effective as monotherapy in patients with active rheumatoid arthritis: results from a randomised, dose-finding study (DARWIN 2).[J]. Annals of the Rheumatic Diseases, 2017, 29 1: 1009-1019. DOI:
10.1136/annrheumdis-2016-210105[4] YIYAN WANG. Oncostatin M inhibits differentiation of rat stem Leydig cells in vivo and in vitro[J]. JOURNAL OF CELLULAR AND MOLECULAR MEDICINE, 2018, 23 1: 426-438. DOI:
10.1111/jcmm.13946