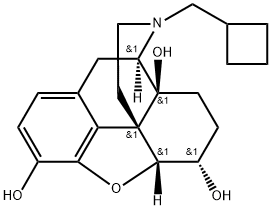
To a slurry of 110.5 g of 14-hydroxydihydronormorphinone in 2.5 liters of
methylene chloride and 280 ml of triethylamine was added a solution of 106 g
of cyclobutanecarboxylic acid chloride in 500 ml of methylene chloride. The
temperature of the reaction mixture was maintained at 20°C to 25°C during
the addition. After 5 minutes the reaction mixture was brought to reflux and
heated for 5 hours.
It was then cooled, washed with water, dried over sodium sulfate and
evaporated to dryness. The residue was crystallized from benzene and
pentane to give 138.5 g of the dicyclobutanecarbonyl derivative, melting point
about 112°C (dec.).
The dicyclobutanecarbonyl derivative (136.7 g) was dissolved in 200 ml of
tetrahydrofuran and added dropwise to a suspension of 34.2 g of lithium
aluminum hydride in 1 liters of tetrahydrofuran. The temperature of the
mixture rose to reflux during the addition. Reflux was maintained for 2 hours
after the addition was completed. After cooling, 110 ml of ethyl acetate was
added dropwise, followed by 30 ml of water, followed by a solution of 53 g of
ammonium chloride in 125 ml of water. The resulting mixture was filtered and
the inorganic precipitate was washed with methanol. Evaporation of the
combined filtrates gave 66 g of N-cyclobutylmethyl-14-hydroxydihydronormorphinone, melting point 229°C to 231°C.