Ethylcentralite (EC), also known as 1,3-Diethyl-1,3-diphenylurea, is a unique compound derived from ethylenediamine, with extensive research focused on its applications in laboratory experiments. The investigation of EC encompasses its potential as an anti-inflammatory, antioxidant, and cognitive enhancer. Additionally, EC shows progression in cancer and neurodegenerative disease research and gene therapy. Furthermore, EC may act as a chelator, effectively binding to metals like iron and copper to prevent their accumulation in the body.
As stabilizer of smokeless explosives.
1,3-Diethyl-1,3-diphenylurea is a gunshot residue and a stabilizer for smokeless powder.
White flakes or white crystalline solid. Peppery odor.
N,N'-DIETHYL-N,N'-DIPHENYLUREA (1,3-Diethyl-1,3-diphenylurea) is an amide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx). N,N'-DIETHYL-N,N'-DIPHENYLUREA is incompatible with acids and oxidizing agents. N,N'-DIETHYL-N,N'-DIPHENYLUREA reacts violently when severely shocked or exposed to extreme temperatures.
ACUTE/CHRONIC HAZARDS: N,N'-DIETHYL-N,N'-DIPHENYLUREA may react violently when severely shocked or heated to extreme temperatures.
N,N'-DIETHYL-N,N'-DIPHENYLUREA is combustible.
Flammability and Explosibility
Non flammable
Poison by
intraperitoneal route. Moderately toxic by
ingestion. Combustible when exposed to
heat or flame. Probably a slight explosion
hazard, although it is a component of
smokeless explosive mixtures. When heated o decomposition it burns and emits very
toxic fumes of NOx. To fight fire, use dry
chemical, CO2, spray or mist. An explosion
regulator.
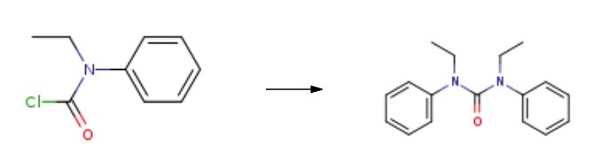
The specific synthesis steps of 1,3-Diethyl-1,3-diphenylurea are as follows: A 10 mL Schlenk tube equipped with a magnetic stirring bar was charged with aryl carbamoyl chlorides and KHCO3, and then Pd(PPh3)4 were added. Finally, 1,4-dioxane was added to the mixture via syringe at room temperature under N2. The tube was sealed and put into a preheated oil bath a 100 °C for 48 h. The mixture was cooled to room temperature, quenched with water, and diluted with ethyl acetate. The layers were separated, and the aqueous layer was extracted with 2 × 5 mL of ethyl acetate. The combined organic extracts were dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo. The crude product was then purified by flash chromatography on silica gel (H), eluting with 30-35% ethyl acetate/petroleum ether.