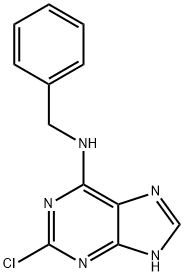
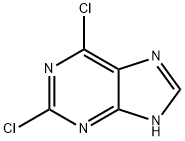
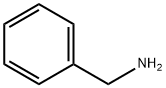
Synthesis of N-benzyl-2-chloro-9H-purin-6-amine (4): 2,6-dichloropurine (110 mg, 0.52 mmol) was suspended in n-butanol (3 mL) and benzylamine (57 mg, 0.52 mmol) and triethylamine (72 mg, 0.79 mmol) were added sequentially. The reaction mixture was heated at 60 °C with stirring for 15 min. Upon completion of the reaction, the precipitate was collected by filtration and washed sequentially with water (20 mL) and methanol (10 mL), followed by drying in air overnight to give off-white solid product 4 (130 mg, 95% yield). The product was characterized as follows: melting point 262 °C; EI/MS m/z (relative abundance): 259 (19, M+), 260 (14), 261 (17%), 106 (100), 91 (77); 1H NMR (400 MHz, DMSO-d6) δ 8.15 (s, 1H), 7.25-7.34 (m, 5H), 4.66 (d J = 6 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 155.0 (s), 153.1 (s), 150.7 (s), 140.2 (d, 1JC-H = 200 Hz), 139.6 (s), 128.5 (d, 2JC-H = 158 Hz), 127.5 (d, 3JC-H = 157 Hz), 127.0 (d, 4JC-H = 158 Hz), 118.1 (s), 43.4 (t, 2JC-H = 139 Hz).