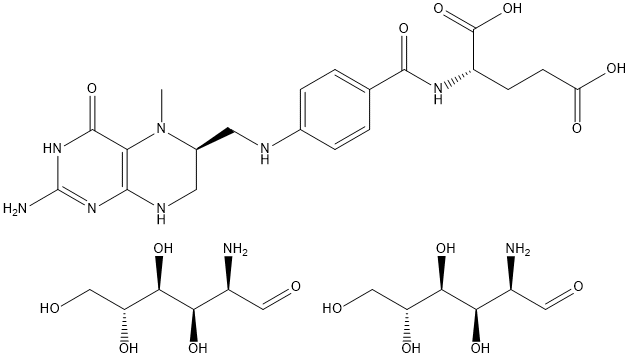
(6S)-5-methyltetrahydrofolic acid, glucosamine salt is chemically synthesised from folic acid and glucosamine hydrochloride and is intended to be used as an alternative source of folate in food supplements. It will be marketed under the trade name of “Quatrefolic”. The calcium salt of folic acid is already authorised as a food supplement in the EU while glucosamine hydrochloride has a history of safe consumption as a food supplement prior to the coming into force of the novel food Regulation (EC No 258/97). The glucosamine hydrochloride used to manufacture the novel ingredient is from a nonshellfish source. The specifications of the novel ingredient and its precursors are provided in detail with a final purity of ≥97.5%. The novel ingredient may contain up to 2.5% impurities, mainly folate-related substances resulting from the breakdown or oxidation of methyl-tetrahydrofolic acid.
GlucosaMine L-5-Methyltetrahydrofolate is used as organic synthesis intermediate and pharmaceutical intermediate, mainly used in laboratory research and development process and chemical production process.