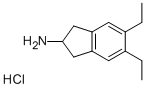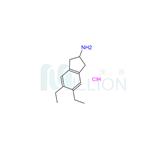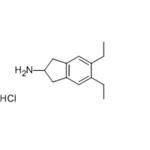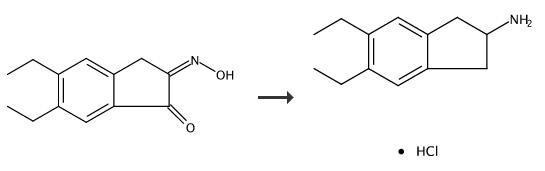
Preparation 4-5,6-Diethyl-indan-2-ylamine Hydrochloride 5,6-Diethyl-3-oxime-1H-indene-1,2(3H)-dione (4.5 g) is added to a mixture of acetic acid (150 mL), and concentrated sulphuric acid (4.5 mL). Pd/C5% (1.5 g) is added, the reaction mixture degassed with nitrogen, and hydrogenated for 5 hours. The catalyst is then removed by filtration, the pH brought to pH 10 with 4M NaOH, and the solution extracted with chloroform. The organic phase is dried with magnesium sulphate, and the solvent removed in vacuo. The residue is redisolved in a minimum amount of ether, and HCl saturated ether added. The white precipitate is filtered and dried to yield the HCl salt of 5,6-diethyl-indan-2-ylamine, a compound of formula XVII where R3, R4 and R7 are H, R5 and R6 are each CH3CH2-, R30 is hydrogen and n is 1. 5,6-Diethyl-indan-2-ylamine Hydrochloride.