Rengasil, Ciba Geigy ,France ,1981
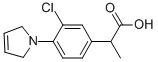
Pirprofen is a non-steroidal anti-inflammatory drug.
ChEBI: Pirprofen is a pyrroline.
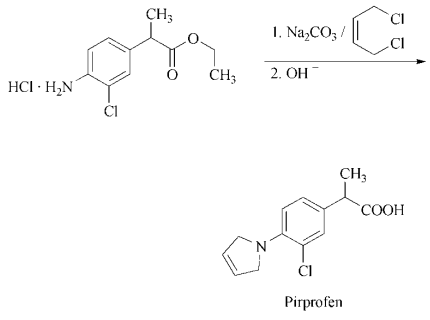
To the mixture of 85.5 g ethyl α-(3-chloro-4-aminophenyl)-propionate hydrochloride, 142 g sodium carbonate and 600 ml dimethyl formamide, 107g 1,4-dibromo-2-butene are added dropwise while stirring and the whole is refluxed for 5 hours and allowed to stand overnight at room temperature. The mixture is filtered, the filtrate evaporated in vacuo, the residue is triturated with hexane, the mixture filtered, the residue washed with petroleum ether and the filtrate evaporated. The residue is combined with 280 ml 25% aqueous sodium hydroxide and the mixture refluxed for 8 hours. After cooling, it is diluted with water, washed with diethyl ether, the pH adjusted to 5 to 5.2 with hydrochloric acid and extracted with diethyl ether. The extract is dried, filtered, evaporated and the residue crystallized from benzene-hexane, to yield the α-(3-chloro-4-pyrrolinophenyl)-propionic acid melting at 94°C to 96°C.
World Health Organization (WHO)
Pirprofen, a nonsteroidal anti-inflammatory agent, was introduced
in 1982 primarily for the treatment of rheumatic diseases, as well as for use in posttraumatic
and post-operative inflammatory conditions, acute gout and
dysmenorrhoea. Reports of serious adverse effects, in particular cases of liver
toxicity, some of which were fatal, led the manufacturer, in 1985 and in 1989, to
amend the approved product information of the drug, limiting duration of treatment
and lowering the recommended doses. In the light of these successive restrictions,
which have considerably reduced the field of application of pirprofen and in view of
available alternatives, the manufacturer has decided to discontinue the drug
worldwide.
Rengasil (Ciba, Greece), Seflenyl (Geigy, Argentina).
Pirprofen has been used to treat rheumatoid arthritis, osteoarthritis, and ankylosing
spondylitis. An optimal dosing regimen of
200 mg three times a day has been developed for
maximal activity with minimal adverse effects.
Pirprofen also is effective in relieving pain from
malignant disease and oral surgery.
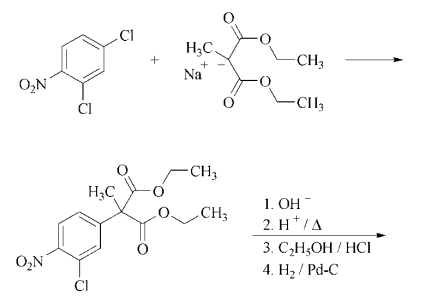
Synthesis: treatment of the sodium
salt of diethyl methylmalonate with 2,4-
dichloronitrobenzene yields diethyl (3-chloro-
4-nitrophenyl)methylmalonate. Saponification,
decarboxylation, and subsequent reesterification followed by catalytic reduction gives ethyl
4-amino-3-chloro-α-methylbenzeneacetate hydrochloride. Treatment of the latter with 1,4-
dichloro-2-butene in the presence of sodium
carbonate followed by saponification affords
pirprofen.