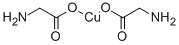Blue, triboluminescent crystals. Slightly soluble in water and alcohol; insoluble in hydrocarbons, ethers, and ketones.
Catalyst for rapid biochemical assimilation
of iron, electroplating baths, photometric analysis,
feed additive.
Copper glycinate has the following benefits:
Helps activate enzymes important to energy metabolism
Assists in the formation of hemoglobin and red blood cells
Supports protein metabolism and phospholipid synthesis
Available in copper citrate with enhanced bioavailability and copper glycinate forms
Made with high-quality vegan ingredients backed by verifiable science
Occurs as monohydrate and as dihydrate. The monohydrate is long deep-blue needles. The dihydrate is light blue powdery crystals.
Salts, basic, such as Copper glycinate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
INHALATION: Inhalation of dust may cause nasal congestion. EYES: Conjunctivitis and edema of eyelids. SKIN: Irritation. INGESTION: Vomiting caused by local irritant and astringent action of ionic copper on stomach and bowel.
Flammability and Explosibility
Not classified
Too much copper can cause nausea, vomiting, stomach pain, headache, dizziness, weakness, diarrhea, and a metallic taste in the mouth. Copper toxicity is rare but can cause heart problems, jaundice, coma, even death. DO NOT use copper supplements if you have diarrhea.
Copper glycinate is an amino acid salt of copper, which can be produced by reacting 0.01 mol/L CuSO4 and 0.02 mol/L glycine under weakly alkaline conditions at room temperature, adding ethanol to precipitate and then recrystallizing with hot water, adding ethanol to precipitate, and vacuum drying. The reaction of copper oxide and glycine also gives the product.