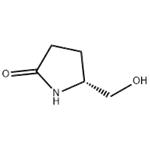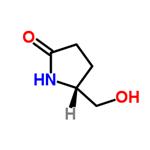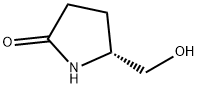
5-Hydroxymethylpyrrolidin-2-one
- Product Name5-Hydroxymethylpyrrolidin-2-one
- CAS66673-40-3
- MFC5H9NO2
- MW115.13
- EINECS626-701-7
- MOL File66673-40-3.mol
Chemical Properties
| Melting point | 83-85 °C(lit.) |
| Boiling point | 147-149 °C0.06 mm Hg(lit.) |
| Density | 1.1808 (rough estimate) |
| refractive index | -31 ° (C=5, EtOH) |
| Flash point | 147-149°C/0.06m |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Chloroform, Dimethylformamide, Water |
| pka | 14.35±0.10(Predicted) |
| form | Crystalline Powder or Flakes |
| color | White to yellow |
| optical activity | [α]20/D 31°, c = 5 in ethanol |
| BRN | 5728422 |
| InChI | 1S/C5H9NO2/c7-3-4-1-2-5(8)6-4/h4,7H,1-3H2,(H,6,8)/t4-/m1/s1 |
| InChIKey | HOBJEFOCIRXQKH-SCSAIBSYSA-N |
| SMILES | OC[C@H]1CCC(=O)N1 |
| CAS DataBase Reference | 66673-40-3(CAS DataBase Reference) |
Safety Information
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36 |
| WGK Germany | 3 |
| F | 3-10 |
| HS Code | 29337900 |
| Storage Class | 11 - Combustible Solids |
| Hazard Classifications | Eye Irrit. 2 Skin Irrit. 2 STOT SE 3 |